Flame retardant solutions for modified plastics: Halogen-Free Mainstream Categories + Emerging Nanotechnology

As environmental regulations become stricter and the market increasingly demands halogen-free, low-toxicity, and low-smoke materials, halogen-free flame retardants represented by metal hydroxides and silicon-based compounds, as well as emerging nanotechnology flame retardants, are playing an increasingly important role.
Today, we will continue our journey by focusing on distinctive types of flame retardants such as metal hydroxides, melamine-based compounds, silicon systems, and expandable graphite. We will delve into their unique mechanisms of action, performance advantages, and innovative application directions, providing you with a more comprehensive reference for material selection.
Types of flame retardants
Metal hydroxide flame retardant
Metal hydroxides are the most commonly used family of halogen-free flame retardants. These mineral compounds are used for:
-
Polyolefin
-
Thermoplastic elastomer
-
Polyvinyl chloride
-
-
Thermosetting plastics
-
Some engineering polymers (such as polyamides)
The flame-retardant formulations they provide can meet appropriate standards for many applications. The combustion products generated by such formulations have low opacity, low toxicity, and minimal corrosiveness.Mixing inorganic hydroxides provides a cost-effective way to achieve low-smoke flame-retardant formulations.
In addition, inorganic hydroxides are easy to handle and relatively non-toxic. Due to considerations of long-term environmental impact, several inorganic hydroxides are replacing halogenated and phosphorus-containing flame retardants, such as aluminum hydroxide and magnesium hydroxide.
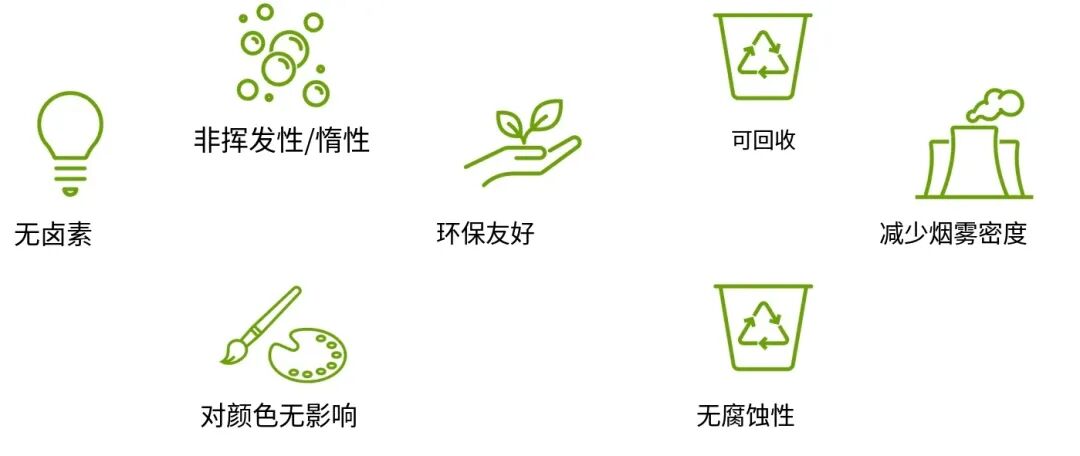
The main advantages diagram of metal hydroxide flame retardants.
Aluminum Hydroxide (ATH)
Aluminum hydroxide is the best-selling inorganic hydroxide used as a flame retardant. ATH is processed at temperatures below its decomposition point (190-230°C, depending on particle size). It is used as a flame retardant for elastomers, thermoset resins, and thermoplastic plastics processed at temperatures below 200°C.
ATH obtained through the Bayer process is a type of trihydrate alumina with a particle size exceeding 50µm, which can be redissolved and precipitated to produce higher purity ATH. Improvements to this process can reduce impurities such as iron, silicon dioxide, or residual solid impurities.
They can be divided into ground hydrates (beige to white, containing sodium silicate, iron impurities, 1.5 to 35µm) and fine precipitated hydrates (white, bright, pure, 0.28 to 3µm). The main differences between different grades of ATH essentially lie in particle size and surface treatment.
Surface treatment aims to enhance one or more specific mechanical properties, such as elongation at break. Fatty acids or metal stearates are commonly used as surface treatment agents for ATH or MH to limit additive agglomeration and improve EB performance in cable and wire applications. There are also some silane-based surface treatment agents with non-reactive (alkyl) and reactive (vinyl, amino, epoxy, methacryloxy) substituents. The type of reactive substituent depends on the polymer using the flame retardant. Silane surface treatments are usually developed for specific applications due to their higher cost compared to fatty acids. Other surface treatments include those centered around elements such as phosphorus, titanium, and zirconium instead of silicon. Titanates and zirconates have specific applications and are generally more expensive than silanes.
Magnesium Hydroxide (MDH)
Magnesium hydroxide is an inorganic flame retardant with higher thermal stability, stable above 300°C, and is used in many elastomers and resins, including engineering plastics and other materials processed at higher temperatures.
It uses different processes to produce from magnesium-containing ores (such as magnesite, dolomite, or serpentine), as well as from brine and seawater. Some ores, such as brucite, hydromagnesite, and artinite, can be directly used as flame retardants or converted into MDH.
The three different processes for producing MDH are: seawater and brine process, Oman process, and Magnifin.®Craftsmanship.
MDH used as a flame retardant is usually of high purity (>98.5%) and most often comes from seawater or brine. Although it is a mineral-derived product, it can also achieve high purity.
Most flame-retardant grades of MDH are white powders with a median particle size between 0.5 to 5 µm. The specific surface area ranges from 7 to 15 m²/g, depending on the shape and size of the particles. Similar to ATH, MDH is used in high loadings, typically between 50% and 70%. The use of MDH as a flame retardant is less common due to its higher cost compared to precipitated grade ATH.
Melamine-based flame retardant
Melamine-based flame retardants represent a small but rapidly growing sector within the flame retardant market. These products offer special advantages compared to existing flame retardants.Cost-effectiveness, low smoke density and toxicity, low corrosiveness, safe operation, environmentally friendly。
In this halogen-free flame retardant family, three chemical groups can be distinguished:
-
Pure melamine.
-
Melamine derivatives (i.e., salts formed with organic or inorganic acids, such as boric acid, cyanuric acid, phosphoric acid, or pyro/polyphosphoric acid)
-
Melamine homologues, such as melem, melam, and melon.
Melamine-based flame retardants exhibit excellent flame retardant properties and multifunctionality due to their ability to employ various flame retardant mechanisms.
The main application areas of melamine-based flame retardants are:
-
Soft polyurethane foam
-
Intumescent coating
-
Polyamide
-
Thermoplastic polyurethane
Through ongoing research and application development, the market for melamine-based flame retardants will expand and, in the near future, will develop towards polyolefins and thermoplastic polyesters.
Silicon-based flame retardant
Silicon-based flame retardants can produce protective surface coatings during a fire, thanks to their low heat release rate. It has been reported that a low content of silicon in certain organic polymer systems can improve their LOI and UL-94 performance.
Some silicone composites (polydimethylsiloxane type) contain dry powders of various organic plastics. In polystyrene, the addition of as little as 1% to 3% can reduce the heat release rate by 30% to 50%. Similar improvements have been reported in HIPS, PS blends, PP, and EVA.
Studies on silicone-modified polyurethane indicate that these materials have a reduced heat release rate compared to unmodified PU. The proposed mechanism is as follows:
-
When burning, a silicon dioxide layer forms on the material surface, which can act as a thermal insulator, preventing energy feedback to the substrate by re-radiating external heat flow.
A novel silicon-based flame retardant for polycarbonate and PC/ABS resins provides good mechanical and high flame-retardant properties, including strength, moldability, and UL-94 1/16 inch V-0 at a 10 phr addition level. Linear and branched types of silicon with or without functional reactive groups (hydroxy or methoxy) have been evaluated. Silicon with a branched structure, aromatic groups in the chain, and non-reactive end groups is very effective. In this case, the silicon disperses within the PC resin and may migrate to the surface during combustion, forming a highly flame-retardant barrier.
Expandable graphite
Expandable graphite provides good flame retardancy at low addition levels and can be used in thermoplastic and thermosetting resins. Expandable graphite can be used alone in natural char-forming polymers (PA, PU, PVC), but is usually combined with other flame retardants such as phosphates, boron compounds, antimony trioxide, or magnesium hydroxide to form a sufficiently strong substrate to support the barrier of expandable graphite. Graphite can also be used in nano-composite PP.

Expandable graphite as a flame retardant for thermoset and thermoplastic plastics diagram
Nanoclay
Nanoclays can reduce relative heat release, promote surface charring, produce anti-dripping effects, and reduce smoke generation. In halogen-free formulations, nanoclays allow for reduced loading levels of mineral flame retardants. In halogen systems, they reduce the required amount of brominated flame retardants or ATO, providing lower density, reduced blooming, and better mechanical properties.
Carbon Nanotubes (CNTs)
Multi-walled carbon nanotubes are commercially used for their antistatic, strength-enhancing, and flame-retardant properties. Their main characteristics include effective char formation; delaying the onset of combustion through heat absorption; increasing viscosity which helps prevent dripping; and not promoting depolymerization. CNTs are expected to find applications in the electronics field, where they can provide both antistatic and flame-retardant properties.

Carbon nanotube structure diagram
Polyhedral Oligomeric Silsesquioxane (POSS)
POSS-based hybrid polymers are fully defined molecules at the nanoscale that can be functionalized with reactive groups suitable for synthesizing new organic-inorganic hybrids. POSS has been successfully incorporated into common polymers through copolymerization, grafting, or blending.
Synthesis of POSS cages, POSS cage-containing monomers, POSS dendritic macromolecular cores, POSS-containing polymers, and POSS nanocomposites can bring specific properties, such as:
-
-
-
-
Viscoelasticity
They are commercially used as flame retardant additives in phenolic resins, PPE, and COC. A key advantage of POSS is its role as a dispersion aid in expanding enhancers and halogen-free flame retardants, which can allow for higher HFFR additive amounts by improving flowability.
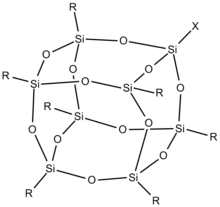
Polyhedral oligomeric silsesquioxane structure diagram
Polymer-clay nanocomposites
Polymer-clay nanocomposites are hybrid organic polymer-inorganic layered materials that exhibit unique flammability characteristics compared to traditional filled polymers. Polyamide-6, polystyrene, and polypropylene are some of the polymers used in conjunction with clay.
Hyperbranched polymer
Hyperbranched polymers, with their numerous ordered branches, pave the way for hyperfunctional additives. They can be prepared from AxBy-type "branched" monomers, where A and B represent functional groups that can react with each other but not with themselves. For the simplest AB2 monomer, the resulting polymer structure is as shown in the figure. The figure considers the advantages of HBP and flame retardant additives, suggesting some possible avenues for developing new flame retardant additives.
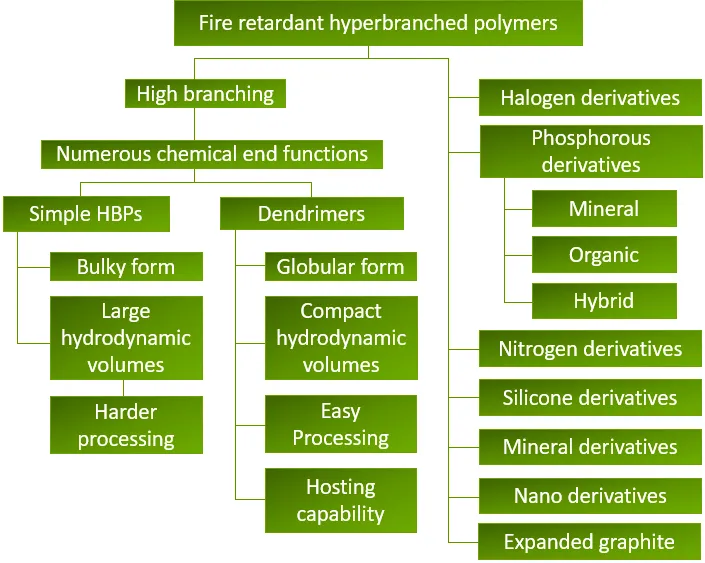
Flame-retardant hyperbranched polymer diagram
Dendritic macromolecules are a special form of HBP, offering additional possibilities. They start from a single focal point or core, with each branch dividing into two (or more) other branches until they reach the terminal functionalized ends. They can accommodate metal atoms to form metallodendrimers. The metals can be located in the repeating units, core, or terminal groups. Metal accommodation extends the performance of metallodendrimers.
Due to its structure, dendritic polymers can exhibit activity at low addition levels. The diagram below compares the structure of hyperbranched polymers (HBP) and dendritic macromolecules with traditional linear macromolecules that have some short side chains.
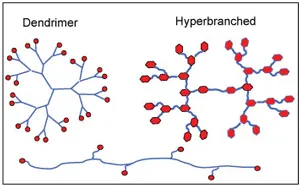
Comparison diagram of structures: dendritic macromolecules (left), hyperbranched polymers (right), and linear macromolecules (below)
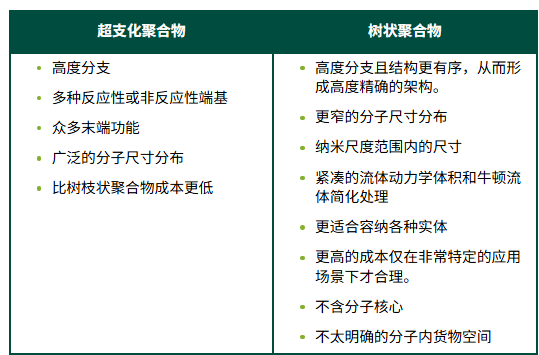
Comparison between HBP and dendrimers
The commercially available Boltorn™ products from Perstorp are produced using a polyol core, hydroxy acids, and technology based on proprietary materials. The dendritic structure is formed by the polymerization of a specific core with 2,2-bis(hydroxymethyl)propionic acid. The resulting base material is a hydroxyl-functionalized dendritic polyester, which is fully aliphatic and composed solely of tertiary ester bonds, reportedly providing excellent thermal and chemical stability.
Various types of different HBPs include polyamides, polyamide amines, polyureas, polyurethanes, polyesters, polycarbosilanes, polycarbosiloxanes, polycarbosilazanes, and many perfluorinated derivatives of the aforementioned polymers. These polymers are suitable for special coating applications, including plastic additives.
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

Clariant Unveils Cost-Cutting Plan Details, Plans to Shut Down Multiple Plants
-

Dow, Wanhua, Huntsman Intensively Raise Prices! Who Controls the Global MDI Prices?
-

[Today's Plastics Market] General Materials Weakly Fluctuate, Engineering Materials Steadily Rise
-

U.S. Appeals Court Officially Rules: Trump Tariff Unlawful and Void!
-

Daily Review: Polyethylene Prices Under Weak Consolidation, Sellers Face Significant Pressure to Move Inventory






