In-depth Analysis! Seven Types of Biomedical Materials for Orthopedics
Bone repair materials mainly refer to materials used for directly supporting, enhancing, or replacing damaged bones. The design and application of these materials focus on the treatment and recovery of bones, such as fracture healing, bone defect filling, and bone strengthening, including naturally sourced bone graft materials (such as autografts and allografts), synthetic ceramics (such as hydroxyapatite and tricalcium phosphate), metals (such as titanium and its alloys), and biodegradable materials (such as polylactic acid and polyglycolic acid).
Orthopedic biomaterials is a broader term that encompasses all biocompatible materials used in orthopedic surgeries and treatments. In addition to including the function of bone repair materials, these materials may also be used to support, replace, or repair other tissues such as tendons, cartilage, and muscles. From fracture repair to joint reconstruction, from cartilage repair to tendon regeneration, each step of treatment may involve one or more specific types of biomaterials.
This article will provide a detailed introduction to seven major orthopedic biomedical materials, which include:
Orthopedic biodegradable internal fixation materials: These materials gradually degrade and are absorbed in the body, avoiding the need for a second surgery to remove them, while providing sufficient initial strength to support bone healing.
# Bone Substitute Materials
Bone substitute materials, also known as bone graft substitutes or bone regeneration materials, are materials used in orthopedic and dental surgeries to replace natural bone. These materials are designed to support, strengthen, or promote the regeneration and repair of damaged or missing bone tissue. The primary function of bone substitute materials is to provide a scaffold structure that promotes the formation of new bone and is ultimately replaced or integrated by the newly formed bone tissue.
The following are some typical products in each category:
 bone screw
bone screw

▲metallic irregular bone plate
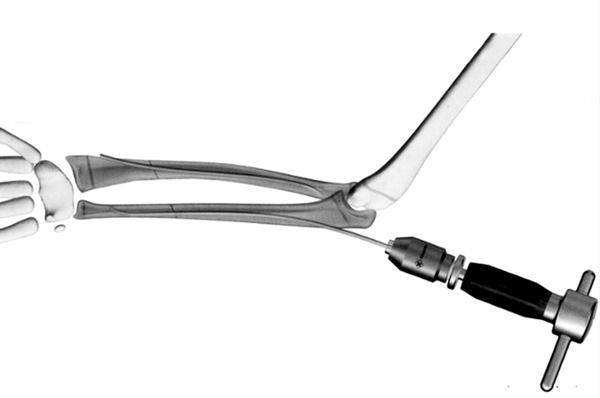
▲General Retrograde Intramedullary Nail System
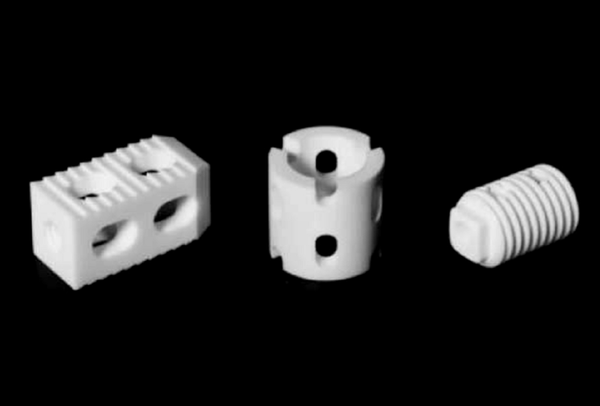
▲different shapes of polyether ether ketone spinal fusion cages
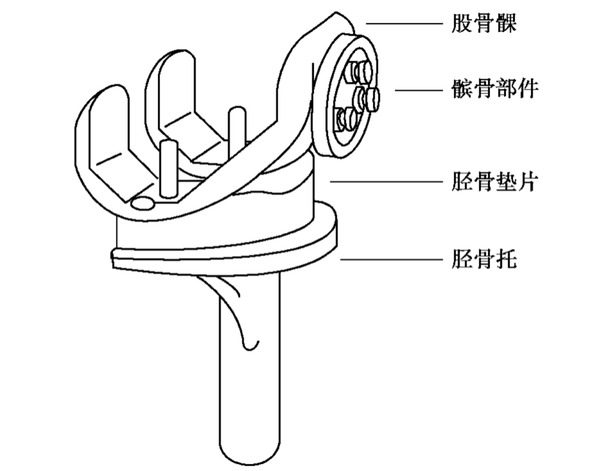
▲Common Total Knee Prostheses
These orthopedic implants have a wide range of clinical applications, providing stable fixation and support to help restore the structure and function of bones. With advancements in biomaterials and manufacturing technologies, the design and materials of these implants are continuously being optimized to improve their biocompatibility, mechanical properties, and ability to promote bone healing.
The main characteristics of bone substitute materials include:
Degradability: Many bone substitute materials are designed to be naturally degraded and absorbed by the body, so as to be replaced over time by newly formed bone tissue.
# Bone Grafting Materials
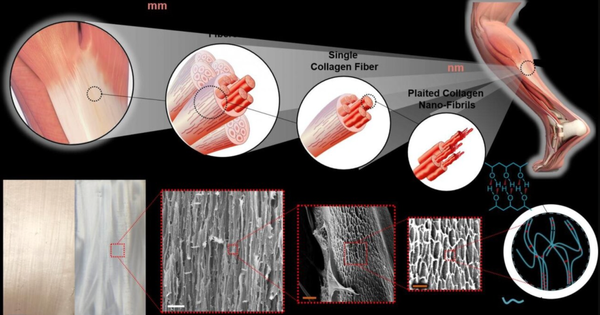
Bone is a natural biological composite material, with an intricate multi-level structure (Figure 4-2-1). Calcium phosphate minerals can account for 60% to 70% of bone weight, and 90% to 95% of the organic phase is collagen, along with small amounts of non-collagenous proteins, polysaccharides, lipids, etc. Bone grafts can be categorized into two major types based on the source of materials: natural materials and synthetic materials; according to the source of the transplanted material, they are divided into autologous bone grafts, allogeneic bone grafts, xenografts, and artificial bone material grafts. In plastic surgery, bone graft materials are mainly used for bone fracture repair, coating of bone implants, revision of artificial joints, and injectable bone graft materials for treating osteoporosis, among other indications. In spinal treatment, they are primarily used for posterior spinal fusion and various vertebral bone defect indications. In dentistry, they are mainly used for tooth extraction trauma and maxillofacial surgery, among other indications.
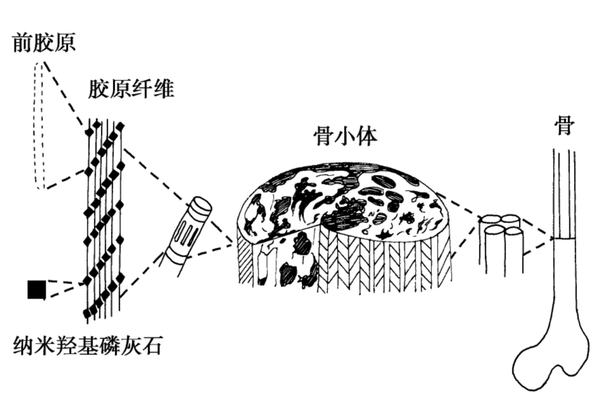
Bone grafting materials are biomedically used materials for repairing or replacing damaged bone tissue. These materials are designed to fill bone defects or provide structural support for bone tissue, promoting bone healing and regeneration. Bone grafting materials can be autologous (from the patient's own bone tissue), allogeneic (from the bone tissue of other individuals of the same species), xenogeneic (derived from other species, such as cows or pigs), or synthetic (artificially manufactured materials).
natural bone graft
Natural bone grafts are commonly used materials in orthopedic and dental surgeries for repairing or reconstructing bone defects and injuries. These materials, sourced from nature, provide good biocompatibility and bioactivity. The following is a detailed introduction to natural bone grafts, including the characteristics and applications of autografts, allografts, xenografts, and naturally derived bone materials.
autologous bone, allogeneic bone, and xenogeneic bone
Autologous bone grafting refers to the process of taking bone from one part of a patient's body and transplanting it to another part. This type of graft is considered the ideal bone graft material because it has the best biocompatibility and osteogenic capacity, and does not cause an immune response. Autologous bone grafts can be further divided into non-vascularized autologous bone grafts and vascularized autologous bone grafts. Non-vascularized grafting procedures are relatively simple and thus have been applied early on; with the advancement of microsurgical techniques, vascularized grafting has developed, which maintains good blood supply to the transplanted bone through vascular anastomosis, preserving the regenerative capacity of the bone, greatly increasing the success rate of the transplanted bone, and improving the quality of autologous bone grafts. The main sources for non-vascularized autologous bone grafts include the ilium, tibia, and calvarial bones; the main sources for vascularized autologous bone grafts include: scapular muscle-skin flap, fibula muscle flap, latissimus dorsi muscle-skin flap, and iliac muscle flap, among others.
Advantages: No immune rejection, high osteogenic capacity.
Disadvantages: Complications such as pain and infection may occur in the bone harvesting area; the amount of available bone is limited.
Allogeneic bone is bone from other individuals of the same species (usually donors). This type of bone graft performs well in terms of osteoconductivity and, after processing, can retain a certain degree of osteoinductivity. Sources for allogeneic bone collection include: (1) bone tissue from amputations; (2) ribs removed during chest surgery; (3) fresh cadaveric bones, including cartilage from deceased infants. It is prohibited to collect bone tissue from patients with tumors, infectious diseases, bacterial infections, bone diseases, or blood disorders. According to the different types of grafts, there are allogeneic bone transplantation, allogeneic cartilage transplantation, and allogeneic bone joint transplantation. Based on the different processing methods, they can be divided into fresh allogeneic bone, banked bone, and human bone matrix gelatin. Allogeneic bone transplantation can avoid some of the disadvantages of autologous bone transplantation, but the main issues are the risks of rejection and cross-infection. Therefore, allogeneic bone must be processed before use, with the aim of reducing or eliminating its immunogenicity. However, after various treatments, the bone cells are damaged to varying degrees, even to the point of death. The biological effect of allogeneic bone in the host site mainly manifests as osteoconduction and osteoinduction. After processing, allogeneic bone is dead bone, and once it comes into contact with the host's bone bed, it is gradually absorbed, with appositional growth on the absorbing bone surface. Through absorption, the allogeneic bone will be "creeping replaced" by the osteoblasts of the host bone and periosteum, thereby generating new bone.
Advantages: Can be used for large segment bone defects, providing good mechanical support.
Disadvantages: may cause immune reactions, risk of disease transmission; requires strict screening and processing.
Processing methods: include fresh bone, deep-frozen bone, and freeze-dried bone. Freeze-dried bone (lyophilized bone) is generally preferred due to its lower immunogenicity.
Xenograft bone transplantation refers to bone material sourced from other species. These materials need to undergo special treatment to reduce immune response and disease transmission risks. The main sources of xenograft bone materials include bovine bone, porcine bone, deer bone, and sheep bone, among which porcine bone and bovine bone are the most readily available and have been the most extensively studied. The current consensus on xenograft bone is that the immunogenicity and inductive activity of xenograft bone share a common material basis. In the process of eliminating antigenicity, the osteoinductive substances are also destroyed, so pure xenograft bone cannot resolve the contradiction between eliminating antigenicity and maintaining inductive activity. Combining de-antigenized xenograft bone with osteoactive substances to create composite xenograft bone can partially restore the osteoinductive capacity of xenograft bone, thus addressing the difficulties posed by this issue to some extent. This has become a new direction in xenograft bone research, such as the combination of xenograft bone with bone morphogenetic protein (BMP), the combination of xenograft bone with autologous red bone marrow, the combination of xenograft bone with bone matrix gelatin, and the combination of xenograft bone with various growth factors.
Advantages: widely sourced, suitable for occasions that do not require high mechanical strength.
Drawbacks: Immune response and biocompatibility issues are more prominent, requiring meticulous handling.
2. Bone-derived materials
In the field of bone repair and regenerative medicine, bone-derived materials play a crucial role, obtained by extracting and processing from natural biological tissues, resulting in materials with specific biofunctionalities. These materials can primarily be categorized into bone scaffold materials and bone matrix materials, each type having its unique advantages and potential application areas. The following is a detailed introduction to these materials:
Calcined Bone (Calcined Bone): Calcined bone is obtained by high-temperature treatment of xenogenic or allogenic bone, removing the organic components (such as fat and protein), mainly leaving the inorganic component hydroxyapatite.
Advantages:
Good biocompatibility: High-temperature treatment thoroughly removes potential antigenic substances.
Excellent bone conduction: retains the microstructure of natural bone, which aids in cell adhesion and proliferation.
disadvantages:
Brittleness: The calcination process may affect the mechanical strength of the material, making it brittle.
Lack of osteoinductivity: high-temperature treatment destroys the bioactive components in natural bone.
Coral Hydroxyapatite (C.HA): Derived from marine coral, it is transformed through physical and chemical methods into a material primarily composed of calcium phosphate and calcium carbonate.
Advantages: Porous structure similar to human bone: Its structure mimics the spongy structure of human bone, which is conducive to the growth of new bone. Suitable for intrabone growth: The pore size is suitable for the inward growth of new bone.
Disadvantages: Limited mechanical properties, although it has a certain compressive strength, its tensile and shear strengths are relatively low.
Demineralized Bone Matrix (DBM): mainly contains decalcified bone collagen and other extracellular matrix. Its advantages are as follows:
Promote bone healing: rich in growth factors and components that promote angiogenesis.
Broad application: commonly used as an adhesive, mixed with other bone substitutes to improve the overall performance of composite materials.
Application: Often used as an autologous bone augmenter or mixed with other materials such as hydroxyapatite.
Decellularized Bone Matrix (DBM): By chemically removing the protein components from xenogenic bone, hydroxyapatite and the natural bone structure are retained. Its advantages are as follows:
Good biocompatibility and mechanical properties: retains the three-dimensional reticular porous system of natural bone.
Low antigenicity: Almost completely removes antigenicity, reducing the risk of immune reactions.
Its disadvantages are as follows:
Lack of osteoinductivity: the processing may destroy active osteogenic substances.
These bone-derived materials each have their unique characteristics and are widely used in orthopedic and dental bone repair and regeneration treatments. Selecting the appropriate material requires consideration of specific clinical needs, expected biological functions, and the patient's specific circumstances. With advancements in materials science, it is possible that more efficient and biologically active new types of bone-derived materials will be developed in the future.
synthetic bone graft
Synthetic bone grafts play an increasingly important role in modern medicine, especially in orthopedic and dental surgeries. The development of these materials aims to mimic the function of natural bone while avoiding some of the limitations and risks associated with autografts and allografts. Synthetic bone grafts can be broadly categorized into inorganic bone graft materials, organic bone graft materials, and composite bone graft materials based on their composition and properties.
inorganic bone graft material
Inorganic bone graft materials are mainly divided into two categories: metal fillers and ceramic fillers. Metal fillers, due to their excellent mechanical properties and ease of processing, are widely used in the manufacture of artificial joints and implant fixtures. Common materials include stainless steel, titanium and titanium alloys, cobalt-based alloys, and nickel-titanium alloys. These metal materials, because of their high strength and good biocompatibility, play a key role in orthopedic implants.
On the other hand, ceramic filler materials are mainly used for bone grafting, including alumina ceramics, hydroxyapatite, and bioglass, among others. These materials not only possess good mechanical properties but also exhibit high inertness to body fluids. In particular, calcium phosphate salt-containing ceramics, such as hydroxyapatite, have received extensive attention and in-depth research due to their excellent biocompatibility and osteoinductive capabilities. Calcium phosphate bioceramics are one of the early widely used bone filling materials, which have been proven to effectively promote bone healing, provide osteoconductive effects, and osteoinductive capabilities.
2. organic bone graft material
In terms of organic bone graft materials, ultra-high molecular weight polyethylene is widely used in wear-resistant implants such as hip and knee joints due to its excellent mechanical properties.
In acrylic materials, poly(methyl acrylate) (PMA) and poly(methyl methacrylate) (PMMA) stand out. Poly(methyl methacrylate) (PMMA) is similar in composition to the commonly used organic glass in daily life. The heat release during PMMA polymerization is significantly higher than that of glass polymers, reaching 78 to 120°C. It is particularly important to protect the tissues it comes into contact with during surgery to avoid thermal damage or even burns that could cause tissue necrosis. PMMA is widely used in joint replacement surgeries for bonding prostheses and autologous bones.
In calcium sulfate bone filling materials, hemihydrate calcium sulfate is often made into an injectable form due to its fast setting speed. When using it, attention should be paid to making the calcium sulfate adhere closely to the viable periosteum or endosteum, so that the calcium sulfate can serve as a matrix for osteoconduction, providing intravascular ingrowth. Calcium sulfate can dissolve and be reabsorbed in the body within 5 weeks. Based on this characteristic, calcium sulfate can be used as a slow-release carrier for antibiotics in the treatment of osteomyelitis.
3. Composite bone graft materials
In the research of composite bone graft materials, mineralized collagen-based composite artificial bone and glass polymer polymers have shown good clinical application prospects.
Composite bone graft materials that have been extensively researched include mineralized collagen-based composite artificial bone and glass polymer polymers. Mineralized collagen-based artificial bone is a type of room-temperature synthesized bone graft product that closely resembles the composition and structure of natural bone, with usage effects similar to autologous bone. This material has a highly porous structure, which facilitates cell attachment and growth. Its main components are Type I collagen and hydroxyapatite crystals. The nanoscale size and specific crystal orientation of these crystals give the material excellent biocompatibility and degradability, while its strength is adjustable, making it convenient for clinical operation and shape customization.
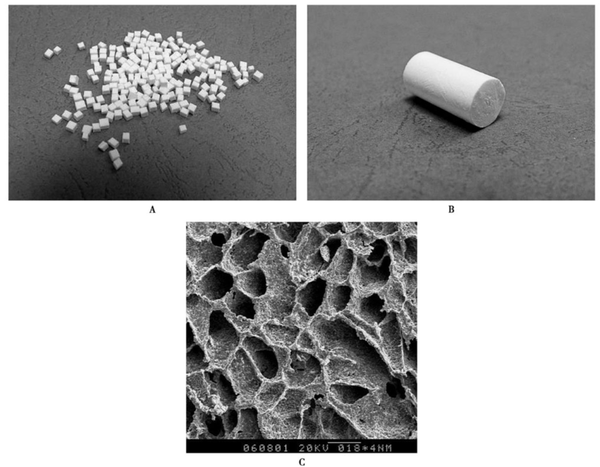
The material has four prominent features:
One is that the material has a porous structure with high porosity, which facilitates cell crawling, attachment, growth, and proliferation, as well as the transport of nutrients. Its mineral phase is hydroxyapatite containing carbonate with low crystallinity at the nanoscale, and it grows uniformly on the collagen matrix. These characteristics, in principle, enable the material itself to have the ability to bond with bone. The surface of the bone implant made from this material can provide a suitable environment for the deposition of collagen and minerals, as well as the adhesion of osteoblasts. Once osteoblasts adhere to the surface of the implant, subsequent bone growth proceeds under cellular regulation.
The second point is that the main components of the material are type I collagen and hydroxyapatite crystals, which can meet the requirements of the physical and chemical properties for the in vivo implantation environment. They have good biocompatibility and degradation performance, with a degradation rate that matches the bone formation rate, and do not cause changes in the pH value of the surrounding body fluid environment during the degradation process.
The third point is that the hydroxyapatite grains in the material are extremely fine, with a scale of nanocrystals, and the C-axis of the hydroxyapatite crystals is parallel to the long axis of the collagen fibers, similar to the structure of natural bone material. In contrast, ordinary hydroxyapatite bone substitutes have larger crystal sizes, making them more difficult for osteoclasts to absorb and degrade. They are also hard to be absorbed and replaced when implanted in the body for a long time, while smaller hydroxyapatite grains make it easier for osteoclasts to absorb and degrade them.
The fourth point is that the strength of the composite material is close to that of cancellous bone and can be adjusted as needed. It can be easily shaped with a scalpel, making it very convenient for clinical use. Clinical use has shown that it has good biocompatibility with the human body, no immune rejection response, and good healing, making it a safe and effective new type of bone graft material.
Calcium sulfate-based composite materials, through the combination with organic polymers or inorganic ceramics, not only improve their mechanical strength but also maintain good biological activity, making them a powerful tool for treating diseases such as osteomyelitis. Mechanical properties refer to the mechanical characteristics exhibited by the material under various external loads (tension, compression, bending, torsion, etc.) in different environments (temperature, medium, humidity). Studies have shown that after calcium sulfate is combined with organic polymers or inorganic ceramics, its mechanical strength can be effectively improved, and it falls between cortical bone (90~230MPa) and cancellous bone (2~45MPa).
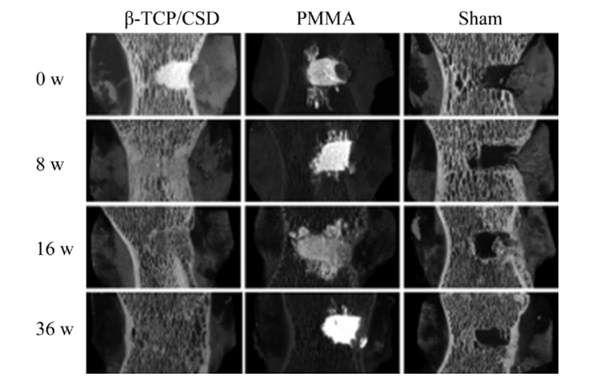
▲Three-dimensional reconstruction images of mature sheep vertebral bone defect filling and repair 0-36 weeks post-operation
Calcium sulfate and organic polymers mainly form stable structures through chemical bonds. In the study by Lewis et al., magnetic resonance detected that -COOH in CS/carmellose composite materials formed new chemical bonds with calcium ions; for different carmellose contents (5%, 7.5%, 10%), the flexural strength of the composites increased by 99%, 103%, and 124% respectively; among them, the compressive strength of the 7.5% and 10% groups increased by 88% and 85% respectively. Gao et al. also found that -COOH formed chemical bonds with calcium ions when CS and polylactic acid were compounded; under a scanning electron microscope, calcium sulfate (with calcium sulfate content below 50%) was uniformly dispersed in the polylactic acid matrix; when the CS content was 40%, the compressive strength reached its maximum, at 82 MPa.
Calcium sulfate and inorganic ceramic materials mainly form a stable structure through physical connections, with different material types, crystal phases, and proportions all affecting the mechanical strength of the composite materials. The structure of calcium sulfate/hydroxyapatite is primarily maintained by the calcium sulfate matrix, with hydroxyapatite simply integrated into it; thus, as the content of calcium sulfate decreases, the mechanical strength will decrease. Among all the crystal phases of calcium sulfate, hemihydrate calcium sulfate can rapidly self-cure through hydration to form dihydrate calcium sulfate with greater hardness, which is of significant importance for early load-bearing in clinical bone defect reconstruction.
Composite materials not only provide structural support and osteoconductivity, but also have a certain degree of osteoinductivity, and perform better in terms of immune rejection. The development and application of these materials have greatly enriched the options for bone repair, enhancing the flexibility and effectiveness of treatment. Through continuous research and technological advancements, synthetic bone graft materials will play an even more critical role in future medical applications.
tissue engineering bone
Tissue engineering bone is an advanced medical technology that combines the principles of biology, engineering, and medicine to construct new bone tissue in vitro for repairing bone defects. This technology mainly involves three core elements: seed cells, biomaterial scaffolds, and growth factors. Seed cells are the basic units for tissue reconstruction, usually derived from the patient themselves or donors, to ensure biocompatibility and reduce immune rejection. Biomaterial scaffolds provide a three-dimensional porous structure that not only supports cell attachment and growth but also helps maintain cell distribution and nutrient transport. Growth factors are key factors in promoting cell proliferation and differentiation, which help accelerate tissue formation and maturation.
The biomedical materials used in tissue engineering can be divided into two major categories: natural biomaterials and synthetic biomaterials. Natural biomaterials such as collagen, hydroxyapatite, and gelatin, due to their excellent biocompatibility and biodegradability, can effectively support cell adhesion, proliferation, and differentiation, and are usually non-toxic and side-effect free to the human body. The main advantage of these materials lies in their ability to provide a biochemical and biophysical environment similar to that of human cells, thereby promoting the formation and integration of new tissues. However, the primary limitations of natural materials are their poor processability and reproducibility, as well as the difficulty in precisely controlling their degradation rate, which may pose certain challenges in clinical applications.
In contrast, synthetic biomaterials such as polylactic acid, polyglycolic acid, and polycaprolactone offer a wider range of options and better processability. The biodegradation rate of these materials can be adjusted as needed, and their mechanical properties can also be designed to meet specific clinical requirements. Synthetic materials are generally less expensive and have better reproducibility, making them suitable for large-scale production. However, their main disadvantage is that compared to natural materials, they have poorer biocompatibility and cell affinity, which may limit their effectiveness in certain clinical applications.
Despite the relatively short development time of tissue-engineered bone, it has already shown great potential for development and application prospects. By optimizing the design of scaffold materials, improving the processing methods of seed cells, and enhancing the application strategies of growth factors, future tissue-engineered bone is expected to play a more important role in the fields of bone repair and reconstruction. The continuous development of this technology not only helps to improve the limitations of traditional bone grafting methods but also may provide more effective solutions for treating complex bone defect cases.
# Cartilage Replacement and Transplant Materials
Cartilage is a special type of connective tissue, composed of cartilage cells (called chondrocytes), fibers, and matrix, with important physiological functions and structural characteristics. The structure of cartilage allows it to withstand pressure and serve as an important component of the skeletal system.
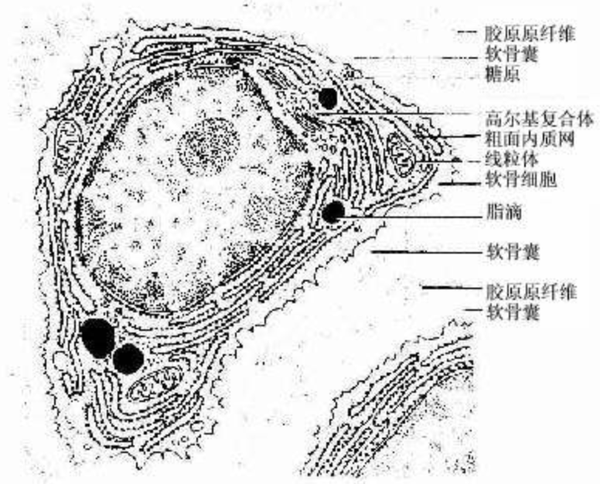
Chondrocytes: Chondrocytes, or chondrocytes, are usually round or oval in shape and are located in small cavities called chondrocyte lacunae. These cells are responsible for forming fibers and secreting the matrix, and they are the active components of cartilage growth and maintenance. The area surrounding the chondrocyte lacunae is rich in chondroitin sulfate and is known as the perichondrium, which helps to protect the cells and facilitate material exchange with the surrounding matrix.
Fibrous perichondrium: The outer part of the cartilage is wrapped in a fibrous perichondrium, which is a tougher connective tissue that can provide additional support and protection. It helps the cartilage to bear loads and connect with adjacent bone structures.
Extracellular matrix: The extracellular matrix of cartilage is its main component, composed of collagen, proteoglycans, hyaluronic acid, as well as liquid components such as water and electrolytes. This complex network not only supports chondrocytes but also provides the essential growth microenvironment for the cells. The high water content of the matrix and the characteristics of hyaluronic acid allow substances to freely permeate through the matrix, providing nutrition to deep chondrocytes even under avascular conditions.
Based on the differences in matrix composition and structure, cartilage can be divided into three types: hyaline cartilage, elastic cartilage, and fibrocartilage.
Overall, the composition and properties of cartilage enable it to effectively withstand pressure and bending while providing crucial structural support in the joints and skeletal system.
In clinical practice, the repair of cartilage defects mainly adopts various methods, including traditional surgical techniques and newer biotechnologies. Traditional methods such as subchondral bone drilling and microfracture techniques promote the natural repair of cartilage by stimulating the release of stem cells from the bone marrow. Debridement and lavage within the joint cavity, as well as joint reshaping, are primarily used to remove fragmented tissue and smooth the joint surface, reducing pain and improving function. In some cases, severe joint damage may require joint replacement to restore joint function.
In biotechnology, tissue transplantation techniques, such as autologous or allogeneic cartilage transplantation, use healthy cartilage tissue to fill in the defective areas. Autologous chondrocyte transplantation is a more refined method, involving extracting cells from the patient's own cartilage, expanding these cells in the laboratory, and then injecting them back into the defective area, usually under the protection of an autologous periosteal flap.
One of the most advanced methods is tissue engineering technology, which combines elements such as seed cells, scaffold materials, and growth factors. By cultivating and constructing specific cartilage tissues in the laboratory and then implanting them into the damaged area, this method not only provides repair materials but also promotes cell proliferation and differentiation through growth factors, thereby accelerating the cartilage regeneration process.
periosteum and perichondrium substitute for articular cartilage
The periosteum is rich in nerves and blood vessels, providing nutritional and sensory functions, and contains multipotent hematopoietic stem cells and mesenchymal stem cells, which have the potential to differentiate into cartilage. Studies have shown that the periosteum, when implanted into articular cartilage defects, can promote the formation of hyaline cartilage and subchondral bone. Despite the advantages of easy access and minimal damage to the body, the clinical application of the periosteum is limited by difficulties in fixation, limited sources, and the inability to fully meet physiological mechanical requirements.
2. Autologous or allogeneic cartilage and chondrocyte transplantation to replace articular cartilage:
Autologous chondrocyte transplantation is a method that involves obtaining healthy chondrocytes from the non-weight-bearing areas of the patient, followed by in vitro culture and expansion, and then implanting them into the damaged cartilage area. This method has been proven to maintain the integrity of the subchondral bone and hinder fibrous repair caused by fibroblasts. Allogeneic chondrocyte transplantation shows similar repair effects to autologous chondrocyte transplantation, but immune response and cell preservation are its main issues.
3. Alternative materials for artificial cartilage:
Artificial cartilage replacement materials should have good biomechanical properties, excellent lubricity and wear resistance, chondrocyte growth induction, and strong bonding with the bone base and biocompatibility. Currently, commonly used highly elastic materials such as silicone rubber, polyurethane, and polyvinyl alcohol hydrogels each have their own advantages and disadvantages, for example, silicone rubber is prone to aging and failure, and the degradation performance of polyurethane needs improvement. The research focus is on improving existing materials and preparation processes, and exploring new materials.
4. Engineered Cartilage:
Tissue-engineered cartilage is achieved through the combination of seed cells and biological scaffolds. The ideal seed cells for cartilage tissue engineering should have the following characteristics:
easy to obtain, abundant in source, with minimal damage to the body
strong ability to proliferate in vitro
has good adhesion to the stent material.
Seed cells implanted in the human body can adapt to the internal environment and maintain the characteristics of the original cartilage cells.
The current research mainly focuses on autologous chondrocytes, allogeneic chondrocytes, mesenchymal stem cells, and embryonic stem cells. The selection of scaffold materials includes both naturally sourced biomaterials and artificially synthesized scaffold materials. The key lies in the design of the scaffold and the correct choice of materials to ensure the mechanical stability of the scaffold and to promote the proliferation and migration of seed cells.
# Tendon tissue replacement and transplant materials
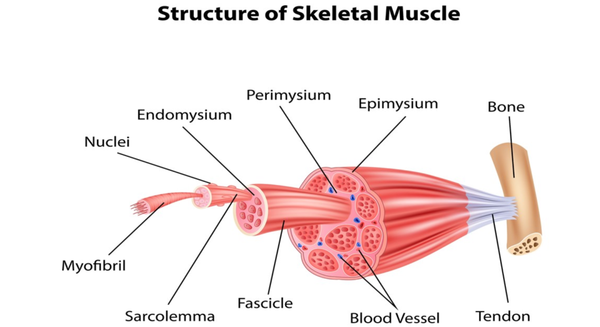
Tendon is a typical regular dense connective tissue, whose main function is to connect muscles and bones, thus transmitting force when the muscle contracts, allowing the bone to move and completing various body movements. The structure and function of the tendon are closely related, mainly consisting of three parts: collagen bundles, endotenon, and tenocytes.
Tendons need to adapt to high tension, and they bear great stretching loads during movement. Overuse, intense exercise, or external accidents (such as cuts, crush injuries) can all lead to tendon injuries. Common injuries include tendon tears or ruptures, which, if not treated in time, may result in permanent functional impairment or disability.
The self-repair ability of tendons is very limited, mainly because the blood supply to tendons is relatively poor, and the repair process is slow and often cannot fully restore the original structure and function. In clinical settings, severe tendon injuries may require surgical repair, including suturing the ruptured tendon or using grafts to replace the damaged part.
Tendon injuries are common sports injuries, especially under conditions of high-intensity or repetitive muscle use. Modern medicine has developed various methods for the repair and replacement of tendon injuries, mainly including autologous tendon transplantation, allogeneic tendon transplantation, xenogeneic tendon transplantation, and artificial tendon substitutes.
autologous tendon graft
Autologous tendon grafting involves using other healthy tendons from the patient for repair. The main advantage of this method is that it avoids immune rejection, as the transplant material comes from the patient themselves. In the early 20th century, Kirschner and other scholars confirmed the feasibility of this method through research on defect repair using autologous tendons. However, the main disadvantage of autologous tendon grafting is the limited availability of tendon resources, and the extraction of tendons may damage the donor site, and sometimes may lead to tears due to insufficient strength at the donor site.
allogeneic tendon transplantation
Allogeneic tendon transplantation uses tendons from the same species but different individuals. This method expands the resources of tendons available for transplantation. However, studies show that this transplantation method has a high failure rate, with main issues including necrosis and rejection of the implanted tendon. Additionally, there is a risk of donor virus transmission, such as hepatitis and AIDS, which limits its clinical application.
allogeneic tendon transplantation
Allogeneic tendon transplantation uses tendons from different species. This method theoretically provides a rich source of tendons, but immune rejection and biocompatibility issues remain the main challenges. Although chemical treatments such as the use of formaldehyde, freezing, and glutaraldehyde can reduce the immunogenicity of the tendon, these treatment methods may alter the biomechanical properties of the tendon, affecting the repair outcome.
artificial tendon substitute
Artificial tendon substitutes cover a wide range of materials, including alloys, plastics, nylon, and synthetic fibers. These materials are designed to mimic the function of natural tendons. However, many attempts have failed due to insufficient mechanical properties of the materials or poor compatibility with surrounding tissues. For example, although carbon fiber artificial tendons were initially considered promising, they were eventually phased out due to issues such as inability to absorb, tensile stress attenuation, and severe adhesion. Currently, researchers are exploring new materials and technologies, such as human hair keratin artificial tendons and tissue-engineered artificial tendons, in hopes of improving the performance and biocompatibility of artificial tendons.
Human hair keratin artificial tendon (HHKAT)
Human hair keratin artificial tendon uses keratin from human hair as raw material, which after special biochemical treatment, forms a biomaterial that can be used for tendon repair. The main advantages of this material include:
The development of this material marks an important advancement in artificial tendon technology, especially in improving the functionality of tendon repair and reducing surgical complications.
2. Tissue-engineered artificial tendon
Tissue-engineered artificial tendons use tendon seed cells and biodegradable materials in a composite manner, which after being cultured in vitro, are implanted into the defective area to promote the proliferation and differentiation of tendon cells, ultimately forming new tendon tissue. The main advantages of this method include:
The key to tissue engineering technology lies in selecting the appropriate seed cells and scaffold materials, as well as effectively combining the seed cells with the scaffold materials. The scaffold materials should not only have good mechanical strength to support early activities but also be able to degrade in sync with cell functions, providing space for cell growth and physiological functions.
orthopedic internal fixation materials
Orthopedic internal fixation materials refer to various medical devices used for internal fixation, which can be implanted in the body to stabilize and support conditions such as fractures, deformities, and bone defects after tumor resection. These materials are designed to withstand the challenges of the in-body environment, such as biocompatibility, corrosion resistance, and sufficient mechanical strength to support the bone healing process. Orthopedic internal fixation materials can be classified according to their type, purpose, and material used. The main types include:
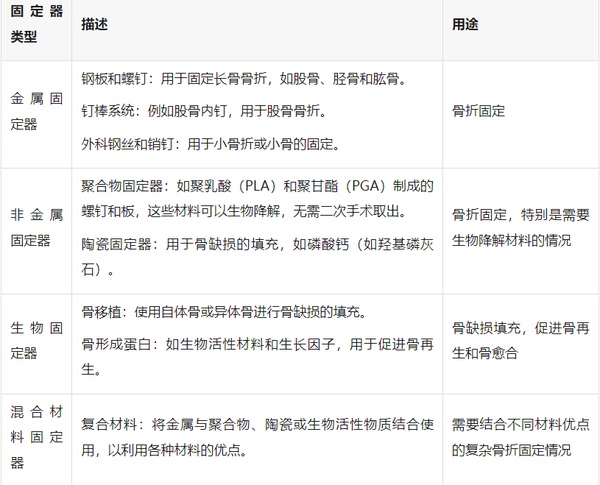
In fracture treatment, internal fixation technology is a key method for restoring stability to the fracture site, allowing for early weight-bearing and limb movement, thereby promoting fracture healing. The selection and application of internal fixation involve multiple factors such as the type of fracture, patient age, and prognosis expectations. For the stability of the fracture, the influence of the following five main forces needs to be considered:
Compressive force: transmitted axially, increasing the load on the bone, commonly seen in compression fractures of the spine.
Tension: also transmitted axially, causing fracture separation.
Bending force: causes one side of the bone to bear pressure while the opposite side bears tension.
Torque: subjecting the bone to rotational force.
Shear force: caused by compressive force, leading to oblique fracture.
To ensure the effectiveness and safety of orthopedic internal fixation materials, the materials used need to meet the following key conditions:
has sufficient strength
Internal fixation devices must have sufficient strength to support the load during the fracture healing process. The characteristics of different materials are as follows:
Degradable materials mainly based on polylactic acid: good biocompatibility, can naturally degrade into water and carbon dioxide after use, eliminating the need for a second surgery to remove. Suitable for internal fixation scenarios that do not bear high loads. The long-term biological safety of its application is still under study.
2. unorganized response
The material should be biocompatible, not causing toxic reactions, inflammation, fibrosis, or macrophage activation. These biological responses may cause pain, swelling, and functional impairment in the implant area, and in rare cases, may even lead to tumor formation. Therefore, for young people, it is generally recommended to remove the internal fixation device once the fracture has healed.
3. does not corrode
The implant should not rust or produce an electrolytic reaction. When using stainless steel, it should be ensured that its purity is high and free of impurities to prevent corrosion. In addition, measures should be taken to prevent contact between different internal fixation materials to avoid interface corrosion. Using internal fixation components of the same material is also an effective way to prevent electrolytic corrosion.
the evolution of internal fixation techniques
The AO/ASIF system is a classic internal fixation system, created by Mueller and other orthopedic surgeons in 1956. This concept primarily focuses on achieving anatomical reduction and stabilization of fractures through internal fixation techniques to promote direct healing of the fracture. The AO team developed a comprehensive internal fixation system, including screws, plates, intramedullary nails, etc., as well as detailed surgical techniques and principles. The core principles of the AO concept include:
anatomical reduction of the fracture ends: especially for intra-articular fractures, it is emphasized that a perfect anatomical reduction must be achieved as much as possible.
Strong internal fixation: providing sufficient stability to meet biomechanical requirements through a precisely designed internal fixation system.
Non-invasive surgical techniques: During the operation, protect the blood supply of the fracture ends and surrounding soft tissues as much as possible, and reduce additional injuries caused by the surgery.
Early activity: Through stable internal fixation support, patients can start muscle and joint activities as early as possible to prevent complications from prolonged bed rest.
AO perspective emphasizes that mechanical stability is the key to achieving fracture healing, and the precision of the operation and the quality of internal fixation directly affect the success of the treatment.
BO perspective, which refers to biological fixation concepts, is a complement and development of the AO perspective. It gradually took shape from the late 20th century to the early 21st century, mainly emphasizing the maintenance or restoration of the biological environment and blood supply in the fracture area during fracture treatment. The BO perspective posits that excessive mechanical fixation may have negative impacts on the biological environment of the fracture site, such as stress shielding leading to osteoporosis, and damage to soft tissues caused by the surgery itself. Therefore, the BO perspective proposes the following principles:
Minimally invasive surgical techniques: Using minimally invasive techniques to reduce the overall impact of surgery on the patient and accelerate recovery.
Minimally invasive internal fixation technique (MIPO): In recent years, the development of minimally invasive surgical techniques aims to reduce damage to soft tissues, thereby protecting blood supply and promoting faster healing.
As the understanding of fracture treatment deepens, traditional views on mechanical fixation are gradually shifting towards biological fixation. Biological fixation takes into account the following points:
Reduce surgical exposure time: Minimize the duration of surgery to lessen the overall impact on the patient.
# Orthopedic External Fixator Bracket and Application
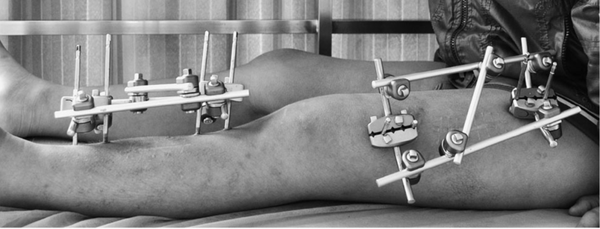
Orthopedic external fixator is a medical device used in fracture treatment and orthopedic surgery, which stabilizes the fractured area or corrects deformities through an external fixation frame. They are fixed to the bones with skin-penetrating needles or pins, and the external structure provides the necessary support and stability to promote fracture healing or correct bone deformities. External fixators are suitable for complex fractures that cannot use internal fixation, infections, or situations requiring progressive adjustments.
External bone fixator is actually a third type of fixation method between orthopedic internal fixation and external fixation, which provides partial immobilization for fractures or dislocations with minimal trauma. It combines the advantages of both internal and external fixation. Compared to internal fixation, it causes less damage and has a lower infection rate. Compared to external fixation methods such as small splints and plaster, it offers more reliable and stable fixation. However, it also has its own limitations and drawbacks, so when choosing to use an external bone fixator, one should strictly adhere to their indications and contraindications.
External fixators are mainly composed of fixation pins, connecting rods, and fixing bolts and nuts. Fixation pins are used to penetrate into the bone to hold it in place, with the pin tails remaining outside the body, connected and fixed by the connecting rods. The main types of fixation pins include Steinmann pins, commonly used for adult lower limb fractures; Kirschner pins, often used for adult upper limb fractures and children's upper and lower limb fractures; half-threaded pins (Schanz pins) with a threaded tip, frequently used for half-pin fixation; and threaded pins with a threaded middle section, typically used for full-pin fixation. Connecting rods serve to connect and stabilize the pin tails, commonly seen in tubular steel, threaded rod, and hook groove styles. Fixing bolts and nuts primarily function to connect and secure the fixation pins and connecting rods.
Orthopedic external fixators can be classified according to their design, function, and purpose of use. The main types include:
unilateral external fixator
Definition: The fixture is located on one side of the fracture, fixing the bone fragments through connecting rods and pin nails.
Application: Suitable for simpler fractures or when local skin conditions do not allow the use of circular or bilateral fixators.
bilateral or multi-lateral external fixator
Definition: The fixture is located on both sides or multiple sides of the fracture, providing more uniform support and stability through multiple connecting rods and pin sales.
Application: Suitable for more stable fixation in complex fractures or reconstructive surgery.
circular external fixator (such as the Ilizarov device)
Definition: A series of ring structures are used to fix fractures or deformities by wires or pins, with the rings connected by rods.
Application: Widely used for complex fractures, long bone lengthening surgeries, or severe deformity corrections. Particularly in pediatric orthopedics, it is used to treat severe limb deformities.
hybrid external fixator
Definition: Combining the characteristics of ring and unilateral or bilateral fixators, utilizing the ring structure to provide stability, while using unilateral or bilateral supports for local adjustments.
Application: Suitable for extremely complex fractures or highly customized deformity corrections.
dynamic external fixator
definition: allow or control the movement of a specific joint while immobilizing the surrounding fracture area.
Application: Mainly used for the treatment of fractures near joints, it can ensure fracture stability while promoting joint function recovery.
When selecting an appropriate external fixator, the following factors need to be considered:
Fracture type and location: Different types of external fixators are suitable for different types and locations of fractures.
The patient's age and health condition: factors such as bone density, skin condition in children and adults may affect the choice of external fixator.
Treatment goal: whether dynamic adjustment or long-term wear is needed, and whether future functional recovery should be considered.
The complexity and maintenance of external fixators: some complex devices may require more frequent monitoring and adjustment by the patient and medical team.
# Orthopedic Biodegradable Internal Fixation Materials
Since the late 1960s, scientists have been exploring and developing the application of biodegradable materials in medicine. Polylactic acid (PLA), as a biodegradable and absorbable material, has achieved certain good results in the surgical treatment of orthopedic diseases due to its excellent biocompatibility, reliable mechanical strength, non-toxic side effects, and convenience of use without the need for a second surgery to remove it. Over the years, the most common absorbable materials on the market have been polylactic acid-based absorbable polyester materials, such as poly-L-lactic acid (PLLA), lactide-caprolactone copolymer (PLGA), and polycaprolactone (PCL). The synthesis principles and pathways of these materials are similar to those of PLA, and different materials can be "hybridized" to form copolymers, thus resulting in a wide variety of final products.
A wide variety of biodegradable screws, nails, rods, bone plates, biological membranes, sutures, intervertebral fusion devices, and other products have been researched and widely used in orthopedic clinical surgeries. Due to their special advantages, they are increasingly being used by orthopedic surgeons to replace traditional metal materials.
absorbable interface screw
In terms of the development of materials science, polylactic acid (PLA) and polymer materials were almost born at the same time. As early as the 18th century in Europe, people isolated lactic acid from fermented milk and obtained the most primitive PLA through direct polycondensation. Lactic acid is a chiral molecule, existing in two optical isomers: left-handed (L) and right-handed (D) lactic acid, which can form four different types of polymers: PDLA, PLLA, PDLLA, and meso-PLA, each with distinct properties. Among them, PDLLA and PLLA are two stereoregular polymers, possessing optical activity, with relatively regular polymer chain arrangements, high crystallinity, and mechanical strength, making them suitable for applications requiring high mechanical strength and toughness, such as sutures, nails, and orthopedic devices. Numerous experimental studies have confirmed that the degradation of PLA is a simple hydrolysis process that does not require the participation of other enzymes; it can be hydrolyzed into lactic acid in the body, entering the tricarboxylic acid cycle, with the final products being water and carbon dioxide, which are metabolized and excreted by the body.
Compared to traditional metal materials, bioabsorbable implants have various advantages; during the degradation process, external force loads are gradually transferred to the bone, effectively avoiding osteoporosis caused by stress shielding; the optimal degradation rate is designed according to the usage of different repair sites, reducing secondary surgical injuries to patients; they have good biocompatibility, are safe and non-toxic, and since polymer materials do not have metallic magnetism, they will not interfere with or affect medical imaging examinations and security checks.
Absorbable internal fixation implant devices have been accepted and recognized by doctors and patients, gradually replacing the use of metal devices, but there are still some issues that need to be resolved and improved:
The strength of absorbable fixation devices is lower than that of metal devices, thus limiting their application range. In the early postoperative period, it is also necessary to cooperate with necessary external fixation. In terms of biomechanics, the yield strength of L-PLA is 70MPa, and the elongation rate is only 5%~10%, which is not sufficient to meet its application in bone tissue repair and surgical sutures. The main defect of bioabsorbable internal fixation materials compared to metal materials is precisely in the mechanical strength and the uncontrollable process of strength attenuation during absorption. Therefore, many scholars have conducted a large amount of research in this area. Since the 1980s, the application of new plastic reinforcement techniques (including self-reinforcement, in-situ synthesis, and stretching, etc.) has led to rapid development in the research of bioabsorbable materials, resulting in a significant increase in effective strength compared to PLLA.
In recent years, to improve the insufficient mechanical properties of PLA, self-reinforcement technology has been invented, which involves sintering suture fibers together under high temperature and high pressure to produce self-reinforced cylindrical PLA rods. Tormala et al. used self-reinforcement technology to produce self-reinforced poly-L-lactic acid (SR-PLLA) rods and screws, with initial bending strength r
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

Key Players: The 10 Most Critical Publicly Listed Companies in Solid-State Battery Raw Materials
-

Vioneo Abandons €1.5 Billion Antwerp Project, First Commercial Green Polyolefin Plant Relocates to China
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics
-

Clariant's CATOFIN™ Catalyst and CLARITY™ Platform Drive Dual-Engine Performance
-

List Released! Mexico Announces 50% Tariff On 1,371 China Product Categories






