Inventory of Bone Substitute Materials
Bone substitute materials, also known as bone graft substitutes or bone regeneration materials, are used in orthopedic and dental surgeries to replace natural bone. These materials are designed to support, enhance, or promote the regeneration and repair of damaged or missing bone tissue. The primary function of bone substitute materials is to provide a scaffold structure that facilitates the formation of new bone, which is eventually replaced or integrated by new bone tissue.
The commonly used materials for orthopedic plates mainly include the following types:
Stainless steel:
With high mechanical performance and low price.
The anti-corrosion ability in the body is generally poor.
Cobalt alloys:
More corrosion-resistant and almost completely inert to tissues.
The mechanical properties are not as good as stainless steel.
3. Titanium alloy:
It has good toughness and fracture resistance.
Good organizational compatibility, elastic modulus close to that of bone.
The price is relatively high.
4. Carbon fiber reinforced polyether ether ketone composite plate (CF-PEEK):
Through technological innovation, carbon within the board layers is embedded in PEEK in different directions to form a new composite material.
The elastic modulus has only slightly increased, but the stiffness and strength have greatly improved.
According to different ratios of carbon fiber, they are divided into CF30, CF50, and CF60, which have been successively applied in the field of spinal trauma and other areas.
Intramedullary nails: used for internal fixation of fractures, especially diaphyseal fractures of long bones, such as V-shaped intramedullary nails, flower-shaped intramedullary nails, and locking intramedullary nails, etc.
Spinal implants: used for stabilization and fusion of the spine, including interbody fusion devices, spinal fixation systems (such as pedicle screw systems), artificial intervertebral discs, etc.
The commonly used materials for spinal implants include the following:
Metal materials
2. Bioactive materials
Calcium phosphate materials: including hydroxyapatite (HA), β-tricalcium phosphate (β-TCP), and a mixture of both (biphasic calcium phosphate, BCP). These materials are commonly used for non-structural bone grafting.
Bioactive glass (BAG): It has good bioactivity and can promote the regeneration of new tissues.
Composite materials such as nano-hydroxyapatite/polyamide 66 composites (n-HA/PA66) and coral hydroxyapatite (CHA) are commonly used in intervertebral fusion devices, offering good biocompatibility and mechanical properties.
3. Polymer Materials
PEEK is currently the best material for intervertebral fusion devices and has to some extent replaced titanium, gaining widespread recognition among orthopedic surgeons. The characteristics of PEEK include a modulus of elasticity close to that of bone tissue, mechanical properties similar to cortical bone, minimal stress shielding, and good radiolucency, which facilitates imaging observation.
Biodegradable materials
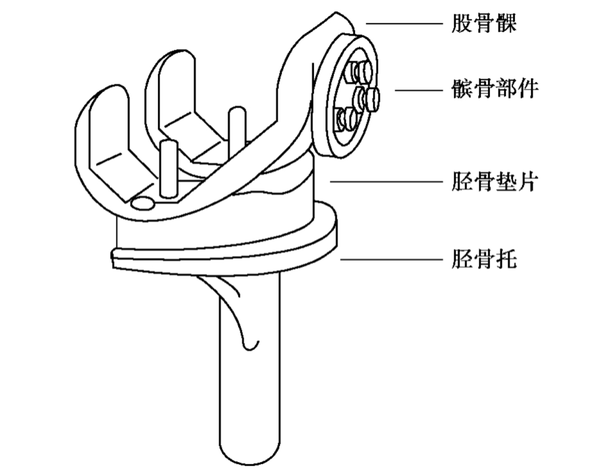
The commonly used materials for artificial joint prostheses mainly include metal materials, polymer materials, and ceramic materials. The following are the specific applications and characteristics of these three types of materials:
Metal materials
Stainless steel: Although it was widely used in artificial joint prosthetics in the early days, it has gradually been replaced by more advanced materials due to the presence of elements, such as nickel, that may cause distortion.
Polymeric Materials
3. Ceramic Materials
These orthopedic implants have a wide range of applications in clinical practice, providing stable fixation and support to help restore the structure and function of bones. With advancements in biomaterials and manufacturing technology, the design and materials of these implants are continually being optimized to improve their biocompatibility, mechanical properties, and ability to promote bone healing.
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

Key Players: The 10 Most Critical Publicly Listed Companies in Solid-State Battery Raw Materials
-

Vioneo Abandons €1.5 Billion Antwerp Project, First Commercial Green Polyolefin Plant Relocates to China
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics
-

Clariant's CATOFIN™ Catalyst and CLARITY™ Platform Drive Dual-Engine Performance
-

List Released! Mexico Announces 50% Tariff On 1,371 China Product Categories






