New chapter! The first commercial implantation of artificial blood vessels has been successfully completed!
March 27, 2025,CIRCULINE®The first commercial implantation of a dialysis artificial blood vessel was successfully completed at the Vascular Surgery Department of Longhua Hospital Affiliated with Shanghai University of Traditional Chinese Medicine, led by Professor Shi Yaxue and her team. This marks the first commercial implantation of this artificial blood vessel since its market launch.Baiyouda and its wholly-owned subsidiary ChangdiUnleashing a new chapter in domestic blood pathway innovation through comprehensive, independent innovation across the entire chain.
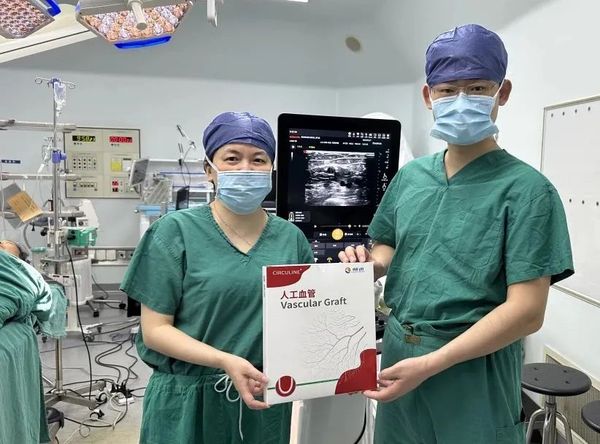
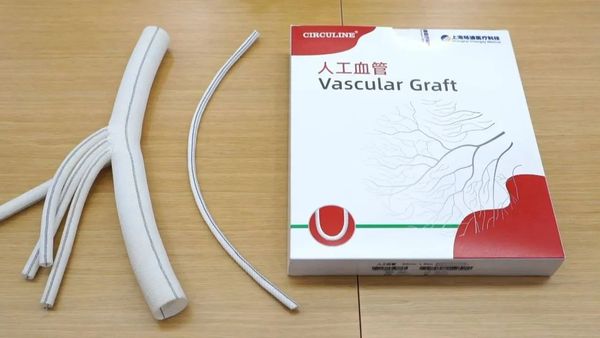
CIRCULINEThe ® dialysis artificial blood vessel received the innovative medical device registration certificate issued by the National Medical Products Administration in December 2024, marking the official birth of China’s first domestically produced dialysis artificial blood vessel approved for market launch.
According to reports, China's special review procedure for innovative medical devices targets products that possess Chinese invention patents, are technologically pioneering domestically and internationally leading, and have significant clinical application value. The approval of the CIRCULINE® dialysis artificial blood vessel for medical device registration signifies that China has broken the import monopoly in the field of dialysis artificial blood vessels.
"Compared to similar products on the market, the CIRCULINE® dialysis artificial blood vessel has undergone disruptive innovation in raw materials and product processes," said Dr. Du Guangwu, the founder of Baiyoda. This product is made of ePTFE artificial blood vessels and textile-type artificial blood vessels combined through medical-grade high-elastic silicone gel, resulting in an innovative dialysis artificial blood vessel with a three-layer structure consisting of inner, middle, and outer layers.
CIRCULINE® dialysis artificial blood vessels are suitable for the establishment of arteriovenous fistulas, creating a vascular access that can withstand repeated punctures and has sufficient blood flow to meet the needs of hemodialysis. This product integrates the advantages of mainstream dialysis artificial blood vessels on the market, providing excellent blood compatibility, biocompatibility, and anti-bending performance, while also being resistant to puncture. Before approval, this product underwent over 300 randomized controlled clinical trials in China, compared to similar products from the American company Gore, which are currently the most used in clinical settings domestically, demonstrating comparable performance in patency rates, anti-thrombosis, and other metrics to imported products.
Jiangsu Biotemed Life Technology Co., Ltd. was established in 2015 and currently has production and R&D bases in Shanghai and Nantong, with over 200 employees. In early 2022, the Biotemed Technology Park, covering an area of 25,000 square meters and located in the Nantong North High-Tech City, began construction. The first phase of the project, a 15,000-square-meter artificial blood vessel production facility, was put into operation in December 2022. The company has set up the Biotemed R&D Center in Zhangjiang Medical Valley, Shanghai, which was officially launched in October 2022. The R&D center's laboratory spans 6,500 square meters and can accommodate the development and production of various products.
The company currently has more than 20 ongoing research projects, covering multiple departments such as cardiovascular surgery, vascular surgery, cardiac surgery, and general surgery. Relying on technical platforms such as polymer materials, biomaterials, and metal stent forming, the company is continuously launching more high-quality products that meet clinical needs, benefiting a wide range of patients.
Since its inception, Baiyouda has consistently focused on the research, development, and production of high-end implantable and interventional medical devices, successively establishing wholly-owned subsidiaries Changdi and Baiheya. Its product line includes VASOLINE.®Artificial blood vessel, VASOPATH®Disposable Strap Retractor, MONOKNIT™Hernia patch, VASO™Non-absorbable surgical sutures and similar products are now on the market.
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

Amcor Opens Advanced Coating Facility for Healthcare Packaging in Malaysia
-

ExxonMobil and Malpack Develop High-Performance Stretch Film with Signature Polymers
-

Plastic Pipe Maker Joins Lawsuit Challenging Trump Tariffs
-

Pont, Blue Ocean Closures make biobased closures work
-

Over 300 Employees Laid Off! Is Meina Unable to Cope?

