Nine Major Adjustments in the Medical Device Industry by 2025, Unveiling Core Transformations
In 2025, the medical device industry is steadily embarking on a journey of continuous transformation and upgrading. This year, a series of significant changes are poised to unfold, signaling that the industry will enter a brand new stage of development. From innovations in payment models to upgrades in regulatory measures, and the deep exploration of value competition by enterprises, every change will profoundly impact the future direction of the medical device industry. Practitioners need to closely monitor these impending changes, seize opportunities, address challenges, and collectively promote the medical device industry towards higher quality and standards.
The collection cycle will see a substantial reduction by 2025. On January 16, the National Medical Security Administration issued the "Notice of the Office of the National Medical Security Administration on Promoting the Reform of Immediate Settlement of Basic Medical Insurance Funds," requiring that by 2025, around 80% of the coordinated regions nationwide basically achieve immediate settlement of basic medical insurance funds with designated medical institutions and pharmacies, and by 2026, all coordinated regions nationwide achieve immediate settlement of basic medical insurance funds with designated medical institutions and pharmacies.
A total of 6 provinces and 76 coordinated regions have been included in the pilot list. The settlement time limit is no more than 20 working days from the day after the designated medical institutions' application deadline to the disbursement of the medical insurance fund.
As of 5:00 PM on January 16, a total of 397 hospitals had settled 2,181 transactions in real time, with funds amounting to 71.97 million yuan; provinces such as Hebei, Zhejiang, Guizhou, Hainan, and Qinghai are also actively promoting this across the entire province.
At the same time, direct settlement between medical insurance and enterprises is also rapidly expanding in the selected products of centralized procurement, with many regions including Fujian, Shandong, Jiangxi, and Guangxi having compressed the reimbursement cycle for pharmaceuticals and medical devices to within 30 days.
"Direct settlement of medical insurance + immediate settlement" working in tandem, coupled with the prepayment fund management system, greatly alleviates the financial pressure on hospitals, and the "triangular debt" issue among medical insurance, hospitals, and pharmaceutical companies is expected to be truly resolved.
According to the National Medical Insurance Administration, by December 2024, 116 coordinated regions had already launched the 2.0 version of the grouping plan ahead of schedule, and the remaining coordinated regions have also completed preparatory work such as detailed grouping and data calculations, switching to the new grouping version on schedule at the beginning of January this year. By March 31 this year, all data working groups in the coordinated regions must be put into actual operation, and the data will be announced to medical institutions.
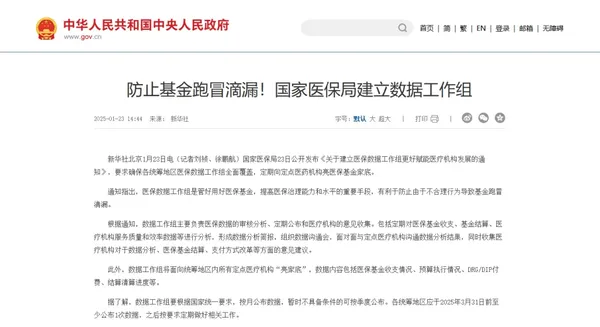
At the same time, the operation of the medical insurance fund has become more transparent this year. On January 23, the National Medical Security Administration issued a notice on "Establishing a Medical Insurance Data Working Group to Better Empower the Development of Medical Institutions," requiring the data working group to "show their cards" to all designated medical institutions within the coordinated region. The data content includes the income and expenditure of the medical insurance fund, budget execution, DRG/DIP payment, settlement and clearance progress, etc. The data working group should publish the data monthly according to the national unified requirements, and those that do not yet have the conditions can publish quarterly.
The regulations clarify the improvement of the management of payment by disease under the "1+3+N" multi-level medical security system, and strengthen the coordination with medical service price reform, centralized bulk procurement, medical insurance catalog negotiation, commercial health insurance, and fund supervision. Promote the coordinated advancement of immediate settlement, direct settlement, and synchronous settlement. Strengthen the coordination and linkage with the high-quality development of public hospitals, the construction of closely-knit county-level medical consortia, and the promotion of mutual recognition of medical examinations and tests.
Each coordinated region conducts review and evaluation based on the standards, procedures, review processes, and settlement methods for special case negotiations. The number of DRG special case negotiations should in principle not exceed 5% of the total DRG discharge cases in the coordinated region, and the number of DIP special case negotiations should in principle not exceed 5‰ of the total DIP discharge cases in the coordinated region.
Special cases can be independently reported by medical institutions, conducted online or offline, on a quarterly or monthly basis, and reviewed and evaluated through a combination of intelligent review and expert review.
On January 5, the National Development and Reform Commission and the Ministry of Finance issued a notice on "Expanding and Extending the Large-Scale Equipment Renewal and Consumer Goods Trade-in Policy by 2025." It clearly states to increase support for key equipment renewal projects. The scale of ultra-long-term special treasury bonds supporting key equipment renewal in important fields will be increased. On the basis of continuing to support equipment renewal in industries such as industrial, energy-consuming equipment, energy and power, transportation, logistics, environmental infrastructure, education, culture and tourism, medical, and old elevators, the scope of support will be further expanded to include electronic information, safety production, and facility agriculture, with a focus on supporting the application of high-end, intelligent, and green equipment.
In addition, conduct in-depth assessments and diagnoses of existing equipment in industries such as industry, agriculture, energy, construction, transportation, education, culture and tourism, and healthcare, and clarify the objectives, tasks, and implementation plans for equipment updates in each sector and industry.
As the large-scale equipment renewal policy continues to advance, the wave of medical equipment updates and iterations will gradually enter the implementation phase, and the relevant market is expected to recover. According to Zhongcheng Data, as of January 5th, the total budget amount for medical equipment renewal tender announcements disclosed in China has reached 8 billion yuan.
Among them, county-level medical institutions may be one of the key focuses for recovery. According to the calculations by Yizhuang Shusheng, as of the end of September last year, the amount for equipment updates in county-level medical consortia that had entered the procurement intention stage accounted for about 22%, and the amount that had entered the bidding stage accounted for about 5.10%; for urban hospitals, the procurement intention was about 17.32%, and the bidding progress was about 4.59%.
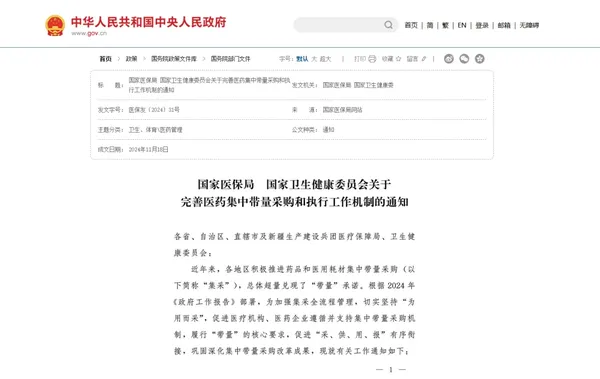
Recent press conferences by the National Medical Insurance Administration have disclosed that in 2025, the medical insurance department will continue to deepen the centralized volume-based procurement of medicines and medical consumables. This includes the national level conducting the 11th batch of medicine procurement in the first half of the year and the 6th batch of high-value medical consumables procurement in the second half of the year. At the same time, it is expected that around 20 specialized national alliance procurements will be carried out at the local level, including traditional Chinese medicine, Chinese herbal decoctions, and high-value consumables.
In addition, the fifth batch of national procurement is expected to implement the selected results for cochlear implants around March 3, 2025 (National Ear Care Day), and the selected results for peripheral vascular intervention consumables are planned to be implemented in May 2025.
In terms of alliance centralized procurement, the national seven major medical consumables joint procurement (Jiangxi biochemical in vitro diagnostic reagents, Anhui tumor markers, Guangdong ultrasonic scalpel heads, Zhejiang breast rotary needles, Fujian vascular tissue closure ligation clips, Hebei vascular intervention, Henan coronary cutting balloons) will be implemented successively. Among them, the joint procurement of Henan coronary cutting balloons also carried out centralized maintenance work in December last year, and it is not far from official launch.
At the fourth plenary session of the 20th Central Commission for Discipline Inspection in January this year, it was emphasized to continuously punish corruption, strictly investigate corruption cases intertwined with political and economic issues, focus on systematic rectification in fields such as finance, state-owned enterprises, energy, fire control, tobacco, medicine, universities, sports, development zones, engineering construction, and bidding, strive to solve the difficulties in discovering, obtaining evidence, and determining the nature of new types of corruption and hidden corruption, seriously deal with the abuse of power, dereliction of duty, and improper decision-making that cause significant losses to state assets, resolutely investigate those who always drag officials into corruption and harm local areas, and increase the efforts in combating cross-border corruption.
It can be foreseen that the pharmaceutical industry and bidding will remain key focuses of anti-corruption efforts this year.
On January 14, the State Administration for Market Regulation issued the "Compliance Guidelines for Pharmaceutical Companies to Prevent Commercial Bribery Risks," consisting of 49 chapters across 4 sections. It covers nine scenarios of commercial bribery risks throughout the entire business, process, and chain in the pharmaceutical purchasing and sales sector, providing specific requirements and prohibitions for scenarios such as academic visits and exchanges, business receptions, discounts, rebates, and commissions, donations, sponsorships, and funding, as well as the free placement of medical equipment.
Recently, the CCTV headquarters aired a TV special titled "Anti-Corruption for the People," which focused on reporting cases related to corruption in the pharmaceutical sector.
The head of the Price and Procurement Department of the National Medical Security Administration, Ding Yilei, mentioned at a recent press conference of the National Medical Security Administration: "The inflated prices did not form corporate profits, were not used for quality, and were not used for the research and development of innovative drugs, but instead ended up in the hidden walls of the homes of corrupt officials as shown in the special documentary by the Central Commission for Discipline Inspection."
On January 11, the National Medical Security Administration issued the "Notice of the National Medical Security Administration on Carrying out the Self-Inspection and Self-Correction Work for the Illegal Use of Medical Insurance Funds by Designated Medical Institutions in 2025," deciding to carry out the self-inspection and self-correction work for the illegal use of medical insurance funds by designated medical institutions nationwide in 2025. The subjects of self-inspection and self-correction include two types of entities: designated medical institutions and designated retail pharmacies. By the end of March 2025, all levels of medical security departments will organize all designated medical institutions and designated retail pharmacies within their jurisdictions to conduct self-inspections and self-corrections on the use of medical insurance funds from 2023 to 2024 based on localized problem lists.
Starting from April 2025, the National Medical Insurance Administration will conduct flight inspections on the self-inspection and rectification status of designated medical institutions nationwide through the "four nos and two direct" method.
For a long time, the prices of medical services have been managed locally by each province, with local pharmaceutical price authorities setting price items and determining price levels. There are significant differences in the number, content, and granularity of price items between regions. In some places, price items are even broken down according to operational procedures, increasing the billing burden on medical institutions. The public finds it difficult to understand, and this system is also unable to accommodate new technologies.
In the second half of last year, the National Medical Insurance Administration made continuous moves in adjusting and managing medical service prices. According to observations, so far, the National Medical Insurance Administration has issued 20 guidelines for the establishment of medical service price projects.
The National Medical Insurance Administration stated that it will continue to accelerate the preparation of project guidelines, aiming to complete the compilation of project guidelines covering most academic fields by the end of 2024, and basically finalize the top-level design for the standardization and normalization of national medical service prices. At the same time, it will guide all provinces to complete the对接落地by the third quarter of 2025. In addition, it will continuously guide local areas in trial operations for 2-3 years, and after revision and improvement, launch a new version of the national medical service price project standard directory at an appropriate time. Note: The phrase "对接落地" seems to be a specific term or concept that might not have a direct translation without more context. I've left it as is, but if you can provide more details or a preferred translation, I can adjust accordingly.
Currently, all over the country, designated medical institutions are accelerating the upload of traceability codes for medical insurance drugs and consumables. On September 30 this year, the National Medical Insurance Administration issued the "Announcement on Further Implementation of Information Collection for Traceability Codes of Medical Insurance Drugs and Consumables," proposing to comprehensively establish a three-code unification mapping library for traceability codes, medical insurance codes, and product codes, as well as a traceability mapping library for large, medium, and small packaging of drugs and consumables, and an identification library for various traceability codes, which will be freely available to designated medical institutions and production and circulation enterprises.
The National Medical Insurance Administration has made it clear that in the future, it will continue to promote the application of drug and medical consumables traceability codes, so that every link from production, transportation to sales can be monitored and recorded in a timely manner. By utilizing big data analysis, problems can be identified promptly, sources traced, and measures taken, ensuring peace of mind for both enterprises and the public.
After the application of drug and medical supplies traceability codes, the return flow, swapping, empty swiping, and counterfeiting in the circulation and retail stages will be thoroughly exposed to big data.
On January 8, 2025, at the national conference on medical prices and procurement, it was clearly stated that efforts should be deepened to manage medical prices, and the supervision and risk management of drug and medical supply prices should be institutionalized and normalized.
The notice from the National Healthcare Security Administration and the National Health Commission on improving the centralized volume-based procurement and implementation work mechanism also explicitly mentioned that for situations where selected or non-selected medical consumables components are combined to form high-priced systems (sets) and used in large quantities, local healthcare security departments should remind medical institutions to standardize their procurement and usage.
Currently, multiple provinces including Hubei, Shandong, Shaanxi, Gansu, and Jiangsu have listed products that have not been subject to time-limited price adjustments in the procurement platform's online directory as suspended from the online directory.
Hubei proposes to regularly carry out centralized volume-based procurement of non-selected medical consumables and their price declarations, with monthly centralized announcements. Products that have been declared and maintained will be marked as "non-selected products" in the online catalog for medical institutions to purchase and use, while other products will be temporarily removed.
National Medical Insurance Administration's Notice on Strengthening and Improving the Credit Evaluation of Medicine Prices and Procurement, it is necessary to reinforce the coordination and linkage between credit evaluation and price management. After determining the inflated price space of a discredited product in one province, other provinces should be promptly notified to pay attention.
As the scope of centralized procurement and price regulation expands, the model of "one place lowers prices, the whole country follows" is accelerating, further squeezing out the inflated water in product prices across different regions.
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

Key Players: The 10 Most Critical Publicly Listed Companies in Solid-State Battery Raw Materials
-

Vioneo Abandons €1.5 Billion Antwerp Project, First Commercial Green Polyolefin Plant Relocates to China
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics
-

Clariant's CATOFIN™ Catalyst and CLARITY™ Platform Drive Dual-Engine Performance
-

List Released! Mexico Announces 50% Tariff On 1,371 China Product Categories