PhD from the Chinese Academy of Sciences: Research and Development of Polyolefins and Their Modification Technologies for High-End Medical Device Applications
Research and Development of Polyolefins and Their Modification Technologies for High-End Medical Devices Applications
Dr. [Name], a senior engineer at the Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, serves as a member of the Surface Engineering Division of the Chinese Mechanical Engineering Society and a committee member of the China Medical Device Industry Association. Focusing on medical devices and energy electronics applications, he has long been engaged in the development of polymer material surface modification technologies. He has led or participated in more than 20 national, provincial, municipal, and enterprise-commissioned projects. He has published over 30 academic papers, holds more than 50 Chinese invention patents and over 20 PCT international patents, and has drafted and revised 2 national standards and 1 industry standard.
In 2020, the first batch of baby boomers born after the founding of New China in the 1960s will officially enter the elderly population phase. As a group that possesses both purchasing power and internet awareness, as well as discerning consumption tastes and quality, they will be officially included in the medical insurance coverage system next year. This holds significant implications for the domestic medical system and the healthcare industry. Currently, with the continuous advancement of technologies such as new materials, 3D printing, and biomimetic materials, the demand for plastics in the medical device industry is also continually increasing.
As early as the last century, most domestic medical devices used plastic materials that were primarily concentrated on disposable plastic medical products made of PVC and PS. Since 2010, with the continuous improvement of the domestic medical system and the internationalization of medical standards, medical plastic products made from PVC have gradually revealed performance deficiencies and have been increasingly replaced by new high-performance plastic materials made from PP.
According to predictions by the China Medical Industry Information Center, the market size of medical devices in China will exceed 600 billion yuan in 2019, with an expected annual compound growth rate of 16.8%. This has been interpreted by the market as a signal that China's medical device industry is about to enter a golden decade. In terms of capital, investments in the medical device industry have already begun to take shape. At the beginning of the year, the three major internet companies, BAT, announced their formal entry into the medical device market, while Huawei Group also started competition in areas such as smart devices and medical internet technology. Driven by capital and technology, it is expected that the medical device market will experience a leap in 2020, and high-end medical devices will gain significant growth potential in this process.
Currently, domestic refining and chemical enterprises mainly focus on increasing the investment and production scale of specialized materials while reducing the proportion of general materials. Leading companies have even exceeded 60% in the production ratio of specialized materials. The production of high value-added specialized materials leads to an optimized production structure and an improvement in the company's sales profit level.
For the downstream demand of the high-end polyolefin products market, approximately 60% of the demand needs to be met through imports, while the self-sufficiency rate for high-end specialized materials is even lower, at less than 20%.
In the next decade, as the proportion of the aging population continues to rise, the era of consumption upgrading for medical-grade polyolefin materials will arrive. Compared to the low profits and low demand of low-end general-purpose materials, the demand for high-end, high-performance polyolefins is likely to continue to grow.
At present, the initial products of medical-grade PE, PC, ABS, and other plastics in our country still mainly rely on imports. However, in the areas of medical-grade PP and PE, some domestic enterprises have made breakthroughs in technology. Companies such as Lanzhou Petrochemical, Beifang JinHua, Maoming Petrochemical, Changling Refining and Chemical, Sino-Korean Petrochemical, and Yanshan Petrochemical have all mastered the key technologies for producing specialized medical materials and high-value-added polyolefin products.
-
In 2014, Beihua Hua Jin Chemical Industry Group Co., Ltd. successfully developed a new transparent polypropylene RP344P-K, which passed the YY/T0242-2007 (Medical Infusion, Transfusion, and Injection Devices Polypropylene Special Material) test. As the first transparent polypropylene in Mainland China to pass this test, it will greatly promote the updating and replacement of medical transparent polypropylene in injection devices in China, and will also better meet the requirements for environmental protection in China, marking a milestone significance.
-
Before 2015, domestic polyolefin pharmaceutical packaging materials relied almost entirely on imports, making the development of high-end medical materials urgent. In 2016, Lanzhou Petrochemical conducted a series of technical breakthroughs and production process modifications in the development of new medical materials, increasing production capacity from 31 tons per hour to 37 tons. RP260 and LD26D medical-grade polyolefin special materials became the high-end brand of Lanzhou Petrochemical's polyolefin products.
-
In 2018, after more than three months of medical device biological evaluation, the medical polypropylene专用料 GA260R produced by Sino-Korean (Wuhan) Petrochemical Co., Ltd. recently obtained a test report issued by a testing institution under the State Food and Drug Administration. The chemical properties comply with the requirements of "Polypropylene Special Material for Medical Infusion, Blood Transfusion, and Injection Devices," and the biological evaluation is qualified, earning the entry pass into the medical industry.
-
In March 2019, Maoming Petrochemical successfully trial-produced a new high-transparency impact-resistant polypropylene product, PPB-MT16, in the chemical distributed polypropylene workshop. This new product is mainly used for manufacturing disposable plastic syringe medical products and features simple packaging, convenience of use, resistance to breakage during use, and ease of processing and molding.
-
In May 2019, the Chemical Department of Changling Refining and Chemical successfully conducted trial production of polypropylene non-woven fabric special material PPH-Y35X using the degradation method at its 100,000-ton polypropylene plant. The product quality meets international standards and can fully replace imported special materials.
-
The medical non-woven fabric dedicated polypropylene resin PPH-Y35X developed by Luoyang Petrochemical has been able to replace foreign brands, and it is also the first Chinese product recognized by the European Nonwovens Association.
-
On October 9, it was reported that the transparent random copolymer polypropylene K4912 produced by Shenhua Coal-to-Oil Yulin Company successfully passed the biological and chemical performance tests conducted by the Guangzhou Medical Device Quality Supervision and Inspection Center of the National Medical Products Administration. The tested product conforms to the YY/T0242-2007 standard for "Special Materials for Medical Infusion, Blood Transfusion, and Syringe Equipment." The successful testing of this product will help promote the upgrade of domestic medical transparent polypropylene in syringe applications, making it more compliant with green and environmentally friendly requirements, and holds significant importance for the differentiated management of the company’s products.
-
Sinopec Shanghai Petrochemical Company recently received RMB 10.8204 million in special financial support funds from the Shanghai Municipal Finance Bureau for five high-tech achievement transformation projects in 2018. The high-tech achievement transformation projects that received municipal support funds include polypropylene special material GM750E for medical infusion bottles, among others. -
Recently, Yanshan Petrochemical's medical polypropylene special material B4902 has passed the associated review by the National Medical Products Administration, becoming the first polypropylene infusion bag material to pass the associated review after the reform of China's new drug approval system. This breaks the long-standing reliance on imports for medical polypropylene packaging materials in China and marks a significant position for Sinopec's medical polypropylene special material in the pharmaceutical packaging market.
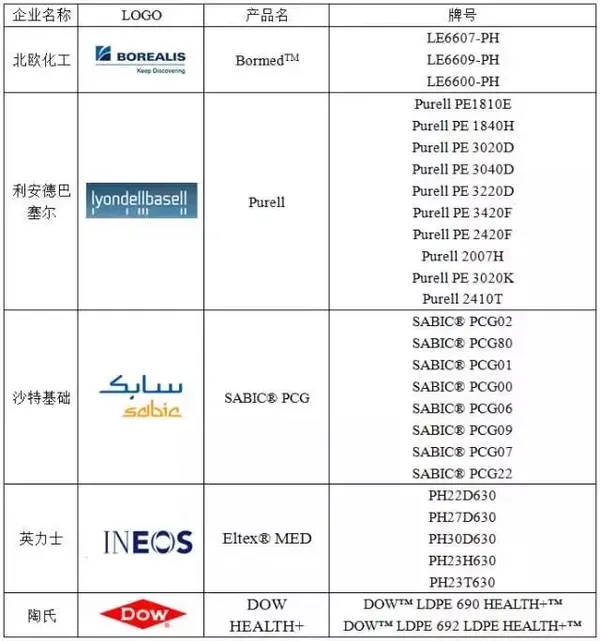
Table 1: Advanced Manufacturers of Medical LDPE Abroad
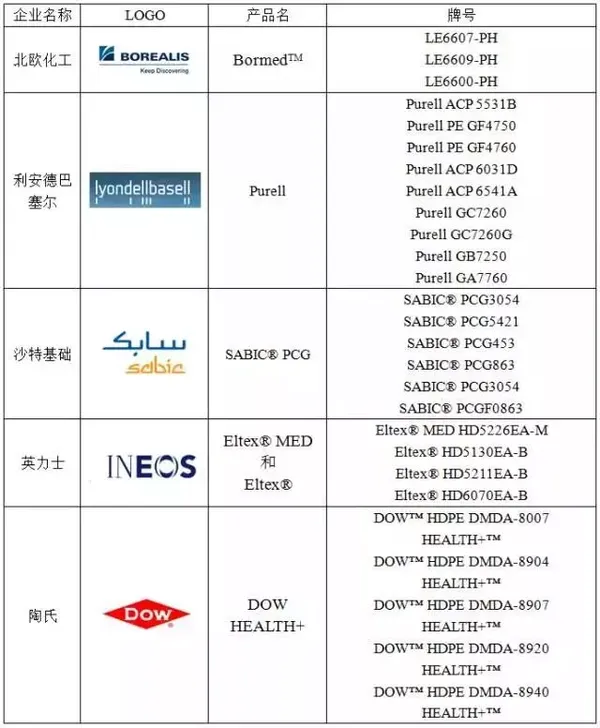
Table 2 Advanced Foreign Manufacturers of Medical-grade HDPE
From the packaging of pharmaceuticals and drugs to the application of disposable medical devices (such as drip bottles, syringes, etc.) and non-disposable medical equipment (such as measuring instruments, surgical instruments, etc.), there are traces of modified plastics.
With the development of technology, the performance requirements for medical plastics used in medical devices and equipment are becoming increasingly stringent, especially in terms of their physicochemical properties and biocompatibility, which must meet relevant international standards (such as those set by the FDA).
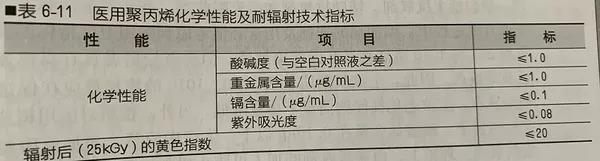
Chemical Properties and Radiation Resistance Technical Specifications of Medical Polypropylene
The current evaluation of the hygiene performance of medical polypropylene in China is mainly conducted according to YY/T 0242-2007 "Polypropylene Special Material for Medical Infusion, Blood Transfusion, and Injection Devices." This standard not only specifies the physical and mechanical properties of medical polypropylene but also its chemical properties and radiation resistance technical indicators.
Medical polypropylene products are mostly sterilized using high temperature or radiation methods, and they also need to have a certain degree of aging resistance. Therefore, stabilizers must be added before processing into products. In the industrial development of resin specifically for medical infusion bottles, the selection and dosage of additives must take into account the requirements of medical hygiene standards.

In addition, according to the requirements for the leachables testing of medical resins, the phosphate content should be below 0.15 μg, and the addition of phosphite ester auxiliary antioxidants is prohibited during production.
In summary, to meet medical hygiene standards, special consideration must be given to catalysts and additives, as well as other factors that may adversely affect hygiene performance. For example, the final stage of the drying unit should use high-purity nitrogen rather than recirculating air as the drying medium; the feeding system for additives must be thoroughly cleaned before and after production; and the filtration cloth in the blending pneumatic conveying system should be regularly replaced.
Medical modified plastics, due to their excellent properties, can replace metals in the manufacturing of large medical diagnostic equipment. For many Chinese manufacturers and exporters of disposable medical plastic products, the importance of the international market is self-evident.
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

Key Players: The 10 Most Critical Publicly Listed Companies in Solid-State Battery Raw Materials
-

Vioneo Abandons €1.5 Billion Antwerp Project, First Commercial Green Polyolefin Plant Relocates to China
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics
-

Clariant's CATOFIN™ Catalyst and CLARITY™ Platform Drive Dual-Engine Performance
-

List Released! Mexico Announces 50% Tariff On 1,371 China Product Categories







