U.S. Department of Health cuts 10,000 jobs and streamlines departments; first automated endoscope cleaning system approved by FDA | Weekly Overseas Medical Device News
【2025W14-Vol.51】
The Trump administration plans to cut tens of thousands of employees from the Department of Health and Human Services.

On March 27, Robert F. Kennedy, the newly appointed U.S. Secretary of Health and Human Services (HHS), announced that the Trump administration plans to cut 10,000 full-time positions within HHS and its subordinate agencies. This layoff initiative is part of a broader federal workforce reduction plan led by the "Department of Government Efficiency" (DOGE), which is headed by Elon Musk.
The institutions facing layoffs include the Food and Drug Administration (FDA), the Centers for Disease Control and Prevention (CDC), the National Institutes of Health (NIH), and the Centers for Medicare & Medicaid Services (CMS). Among them, FDA will cut 3,500 employees, approximately 19% of its total workforce; CDC will cut 2,400 employees, accounting for 18%; NIH will cut 1,200 employees, accounting for 6%; CMS will cut 300 employees, accounting for 4%.
In addition, the HHS also plans to merge the department's 28 bureaus into 15 and reduce its 10 regional offices to 5.
2. FDA Approves First Automated Endoscope Cleaning System

On March 20, the U.S. FDA officially approved the first automated endoscope cleaning system using Nanosonics CORIS technology. Developed by the Australian medical technology company Nanosonics, the system focuses on deep cleaning of the complex internal channels of flexible endoscopes such as gastroscopes and colonoscopes.
Traditional manual cleaning methods have difficulty reaching the narrow, complex internal channels, often leading to the accumulation of biofilm residues, which increases the risk of hospital-acquired infections. The CORIS system utilizes a unique automated cleaning mechanism that can penetrate the narrowest parts of endoscopes, effectively removing tough biofilms.
The FDA has previously urged the use of disposable components or systems to reduce infection risks, but disposable systems are costly and have many limitations. The emergence of the CORIS system provides medical institutions with an economical and efficient solution.
3. Bausch + Lomb recalls some intraocular lens products
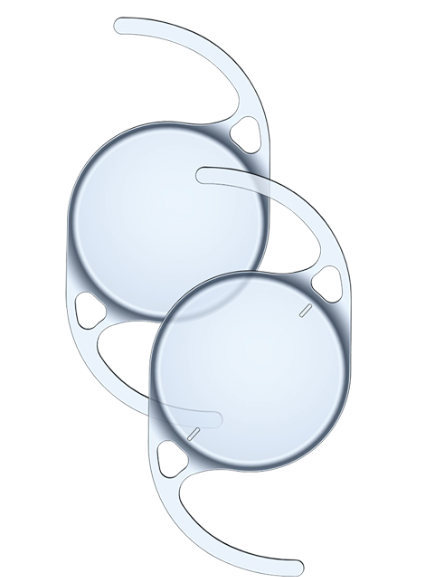
On March 27, Bausch + Lomb announced a voluntary recall of certain intraocular lenses from the enVista platform, including enVista Aspire, enVista Envy, and certain enVista monofocal lenses.
This recall is due to an unexplained increase in cases of toxic anterior segment syndrome following the implantation of these lenses. This condition is an inflammatory reaction in the eye that can occur 12 to 48 hours after cataract surgery, caused by various factors including bacterial contamination or toxic substances during the procedure.
Supira Medical Completes $120 Million Series E Financing to Advance Clinical Programs

On March 26, vascular interventional developer and manufacturer Supira Medical announced the successful completion of an oversubscribed Series E financing round, raising a total of $120 million. The funds will be used to advance clinical programs for the next-generation percutaneous ventricular assist device (pVAD) in high-risk percutaneous coronary interventions and cardiogenic shock, including supporting the U.S. pivotal SUPPORT II study for FDA premarket approval (PMA).
The company has recently completed the enrollment for the U.S. SUPPORT I Early Feasibility Study (EFS), recruiting a total of 15 patients across four clinical centers to evaluate the safety and feasibility of the company's innovative pVAD in high-risk coronary intervention patients.
5. The U.S. Senate confirms the appointment of a new FDA commissioner.

On March 26th, the U.S. Senate confirmed Dr. Martin Makary as the Commissioner of the U.S. Food and Drug Administration (FDA) with a vote of 56-44.
Dr. Makary is a surgical oncologist and gastrointestinal laparoscopic surgeon at Johns Hopkins Hospital, who has long advocated for innovative technologies in the medical device industry. He has also drawn attention for his skepticism toward public health measures during the pandemic, as while he supports vaccination, he holds reservations about childhood vaccinations and long-term lockdown measures.
Dr. Makari stated at the confirmation hearing that he believes in the importance of collaborating with the medical device and pharmaceutical industries, advocating for more independent scientific reviews to promote more localized treatment solutions. He also mentioned that he hopes to put more medical technologies into the hands of consumers, such as promoting over-the-counter drugs and continuous glucose monitoring devices.
Medtronic's Affera ablation mapping system makes its first clinical application in Sweden

On March 26, Medtronic announced that the Affera Mapping and Ablation System was introduced into clinical practice for the first time in Sweden. The system includes the Sphere-9 catheter and the Affera Prism-1 mapping software, designed to provide real-time feedback through intuitive mapping and navigation software, thereby more effectively treating arrhythmias such as atrial fibrillation (AFib).
Solventum and SprintRay Form Strategic Partnership to Advance Dental 3D Printing Technology

On March 26, Solventum announced a strategic partnership with SprintRay, aiming to provide innovative solutions for dental clinics through 3D printing technology.
This collaboration will focus on the development and commercialization of high-quality, durable, and permanent same-day repair solutions, including dental crowns, inlays, and veneers. The goal of the partnership is to launch the first permanent chairside 3D printed dental crown on the market.
Solventum has several products that are widely used in dental restorations. SprintRay, on the other hand, is renowned for its innovative chairside 3D printing ecosystem, capable of providing a one-stop solution for dental restorations.
Evident Vascular Completes Series B Financing to Advance AI-Driven Intravascular Ultrasound Platform
On March 25, Evident Vascular announced the completion of its Series B financing. Although the company did not disclose the specific amount of the financing, the funds will be used to accelerate the development of its AI-driven intravascular ultrasound (IVUS) platform to obtain FDA approval and prepare for launch in the U.S. market.
Evident Vascular's next-generation IVUS platform combines artificial intelligence technology to optimize vascular imaging for peripheral and coronary interventional treatments. The company plans to expand the clinical applications of IVUS through innovation, enhancing its practicality and accessibility.
Terryford announced that the UroLift system for prostate elevation is superior to the Rezūm water vapor therapy.
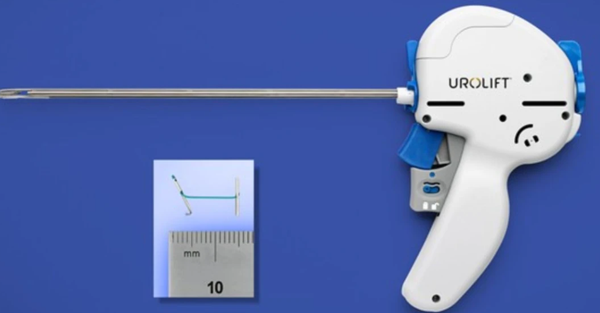
From March 21 to 24, at the 40th European Association of Urology Congress held in Madrid, Teleflex (NYSE: TFX) announced new data from the CLEAR study, showing that the UroLift System outperformed the Rezūm therapy in the clinical treatment of benign prostatic hyperplasia.
This study is the first head-to-head randomized controlled trial (RCT) comparing the UroLift Prostatic Urethral Lift System (PUL) and Rezūm Water Vapor Thermal Therapy (WVTT) for the treatment of benign prostatic hyperplasia (BPH). The results show that the UroLift system performs better in early patient satisfaction and sexual function.
Abbott's IVL System Receives FDA IDE Approval to Initiate Clinical Trials

On March 24, Abbott announced that the Coronary Intravascular Lithotripsy (IVL) System had received a Investigational Device Exemption (IDE) approval from the U.S. FDA. The system is designed to treat severe calcification in coronary arteries using high-energy acoustic pressure waves, preparing the way for subsequent stent implantation. Abbott plans to conduct a clinical trial called TECTONIC, which will recruit up to 335 participants at 47 sites across the United States.
11. Newronika's AlphaDBS system receives CE approval for Parkinson's treatment.

On March 24, neurotechnology company Newronika announced that its AlphaDBS device has received CE mark approval for the treatment of Parkinson's disease.
AlphaDBS is a next-generation closed-loop deep brain stimulation (DBS) system capable of dynamically adjusting stimulation based on real-time brain signals. The system optimizes symptom control, reduces side effects, and minimizes the need for frequent programming adjustments by neurologists by monitoring the patient's brain activity.
Newronika plans to launch the system in certain European markets by 2025 and has obtained the FDA Investigational Device Exemption (IDE) to initiate a pivotal clinical trial.
12. Imperative Care's Symphony 16 Fr 82cm Catheter Receives FDA Approval for Venous Thrombosis Treatment
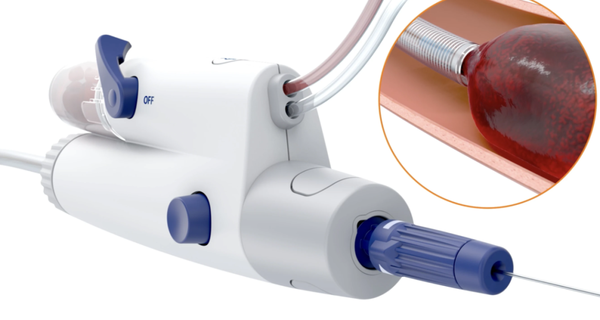
On March 24, Imperative Care announced that the Symphony 16Fr 82cm catheter received 510(k) clearance from the U.S. FDA for the treatment of venous thrombosis.
The catheter is part of the Symphony Thrombectomy System, which also includes 16Fr 117cm and 24Fr 85cm catheters. The system achieves efficient thrombus removal through a large-bore suction catheter and powerful deep vacuum technology, while minimizing blood loss.
Additionally, Inari Medical filed a patent infringement lawsuit in May 2024 against Imperative Care and its subsidiary Truvic Medical, alleging that their Symphony thrombectomy system infringed on Inari's patents. Imperative Care denied the infringement claims and filed a counterclaim. The lawsuit is still ongoing.
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

Key Players: The 10 Most Critical Publicly Listed Companies in Solid-State Battery Raw Materials
-

Vioneo Abandons €1.5 Billion Antwerp Project, First Commercial Green Polyolefin Plant Relocates to China
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics
-

Clariant's CATOFIN™ Catalyst and CLARITY™ Platform Drive Dual-Engine Performance
-

List Released! Mexico Announces 50% Tariff On 1,371 China Product Categories






