Zhejiang University Wang Yong's Team Angew: Plastic Degradation Coupled with Fischer-Tropsch Process for Low-Temperature Efficient Production of Light Aromatics from Polyolefins
Recently, Professor Wang Yong's team from Zhejiang University innovatively proposed a new reaction pathway: using a Ru/Nb2O5 and ZSM-5 catalytic system in a syngas atmosphere to combine the traditional pyrolysis of polyethylene (PE) with the key step of CO insertion mechanism in Fischer-Tropsch synthesis. Under mild conditions as low as 280°C, the yield of light aromatics can reach 67%. The insertion of CO plays a crucial role, as it can mediate the Ru-alkyl species formed during the pyrolysis of polyolefins, promoting the generation of active oxygenates and olefin intermediates while precisely controlling the carbon chain length. This process not only reduces the energy barrier for subsequent aromatization reactions but also significantly improves the selectivity for aromatics, achieving efficient and low-temperature upgrading and conversion of polyolefins.
Globally, over 360 million tons of plastic waste is produced each year, with approximately 79% ultimately ending up in landfills or natural environments, causing severe pollution to soil and oceans, and potentially posing health threats to humans in the form of microplastics. Conventional methods of plastic disposal, such as incineration, are not only high in carbon emissions but also economically inefficient. Particularly for the most common polyolefin plastics (such as polyethylene and polypropylene), due to their highly stable chemical structures, they typically yield a wide distribution of mixed alkanes/alkenes after thermal cracking or hydrogenolysis, making it difficult to achieve high-value utilization.
Converting waste polyolefins into light aromatics (BTX) efficiently not only allows for precise control over product distribution but also significantly increases the added value of the products, enabling them to re-enter the aromatic cycle and extend the material's lifecycle. However, traditional aromatization processes, such as catalytic reforming of naphtha, typically require temperatures above 500°C, which consumes a large amount of energy and can lead to uncontrollable radical cleavage of polyolefins. Therefore, developing a low-temperature, efficient catalytic conversion strategy to break the inert chemical bonds of polyolefins and achieve selective aromatization has become a key challenge in the resource utilization of plastics.
Our research initiative stems from addressing the dual challenges of global plastic pollution and the shortage of petroleum energy. Traditional catalytic cracking or hydrolysis methods for polyolefins (such as polyethylene) predominantly yield widely distributed alkanes or alkenes, which are difficult to utilize at high value-added levels. However, if we could obtain light aromatics (such as BTX), not only could we more precisely control the carbon number distribution of the products, but we could also enhance the added value of these products, enabling them to re-enter the aromatic cycle as major chemical products. This would significantly extend the lifecycle of these products.
Figure 1 summarizes the existing PE recycling technologies and highlights the uniqueness of the new method proposed in this study for the efficient conversion of PE into high-value light aromatics under mild conditions. Through the analysis of the production volume of bulk chemicals and their downstream products, the concept of polyethylene recycling into the aromatic cycle is proposed, providing a new approach to solving the problem of plastic waste.
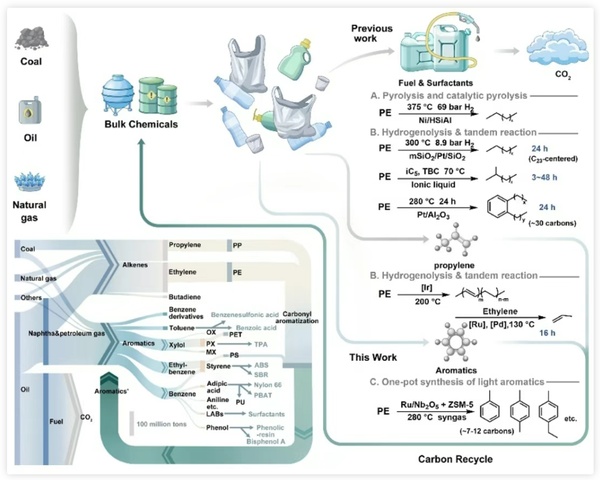
Figure 1. Comparison of recycling routes for waste polyethylene and analysis of production volumes of bulk chemicals and their downstream products
Figure 2 illustrates the synergistic effect of Ru-based catalysts and ZSM-5 catalysts, achieving efficient low-temperature catalytic upgrading of polyolefins. The reaction process can be divided into the following three key steps:
1. Catalytic cracking: Polyolefins undergo cleavage at the acidic sites of ZSM-5, forming lower molecular weight alkanes and alkenes.
CO insertion: The cleavage products are activated at the Ru catalytic site, forming Ru-alkyl species. Subsequently, CO inserts to generate active oxidized products and long-chain olefin intermediates.
3. Aromatization: The active intermediate is further converted into light aromatics under the catalysis of ZSM-5.
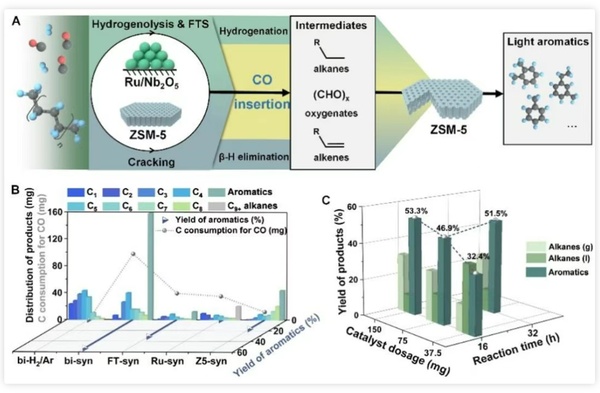
Figure 2. Low-temperature high-efficiency aromatization of PE
Figure 3 verified the reaction mechanism of CO insertion through isotope labeling experiments, demonstrating that CO indeed enters the aromatic products and does so via an insertion mechanism rather than through a methanol pathway. In addition, in the presence of polyethylene (PE), the consumption rate of CO significantly increases, attributed to the optimization of reaction thermodynamics. Specifically, the activation energy for CO insertion into Ru-alkyl is reduced to 1.14 eV, effectively bypassing the slow induction period commonly seen in traditional Fischer-Tropsch synthesis, greatly enhancing reaction efficiency and achieving more efficient upgrading conversion of polyolefins.
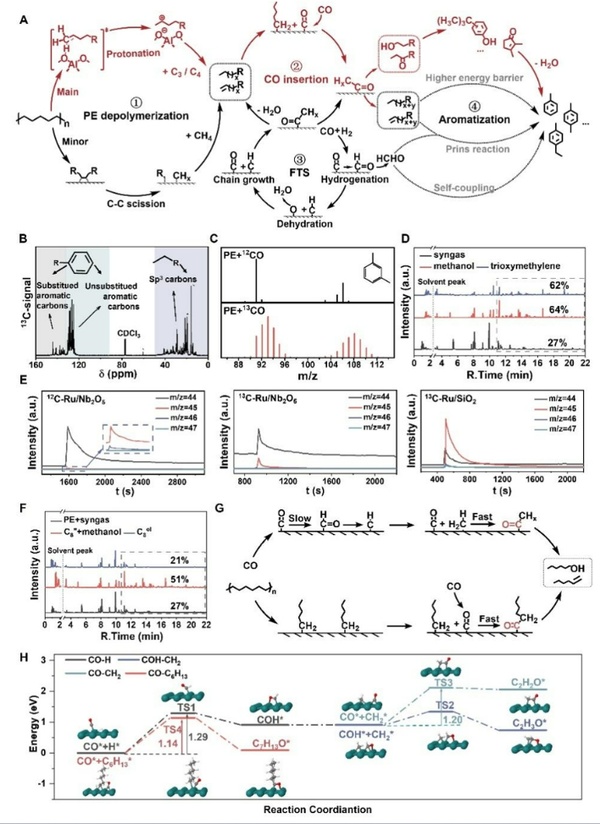
Figure 3. Involvement of CO in the degradation process of PE.
Figure 4 shows the investigation of different catalyst ratios and the study of reaction time curves, which further clarifies the reaction mechanism and the respective roles of the dual catalysts. The existence of oxygen-containing intermediates was confirmed by comparative experiments and the detection of hydrocarbon pool species in the molecular sieve after the reaction. Extending the substrate to short-chain alkanes, it was found that this carbonylation scheme is also applicable and can promote their low-temperature aromatization to a certain extent.
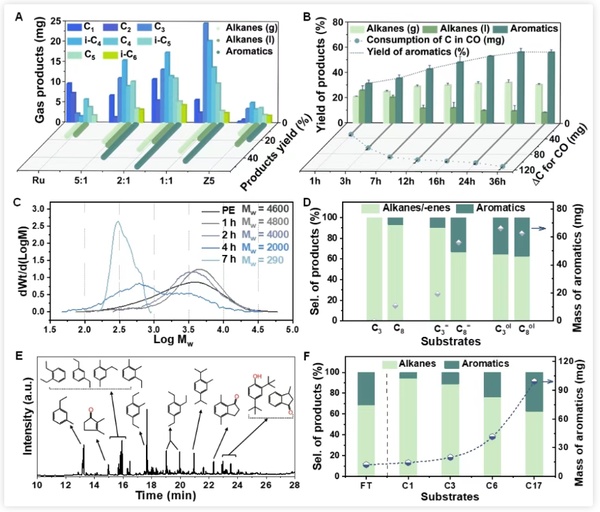
Figure 4. Time course and mechanism of PE low-temperature aromatization cracking
Figure 5 highlights the advantages of the low-temperature aromatization process demonstrated in the techno-economic analysis:
·Wide application: Not only suitable for commercial waste polyethylene, but also extendable to polypropylene and polystyrene and other polyolefins, enabling broader plastic upcycling.
·High economic efficiency: Even without government subsidies, the break-even point is only 3,500 tons, with a static investment payback period of just 3.6 years, giving it strong market competitiveness;
·Energy Saving and Emissions Reduction: Compared to traditional processes, carbon emissions are reduced by about one-third, contributing to green sustainable development.
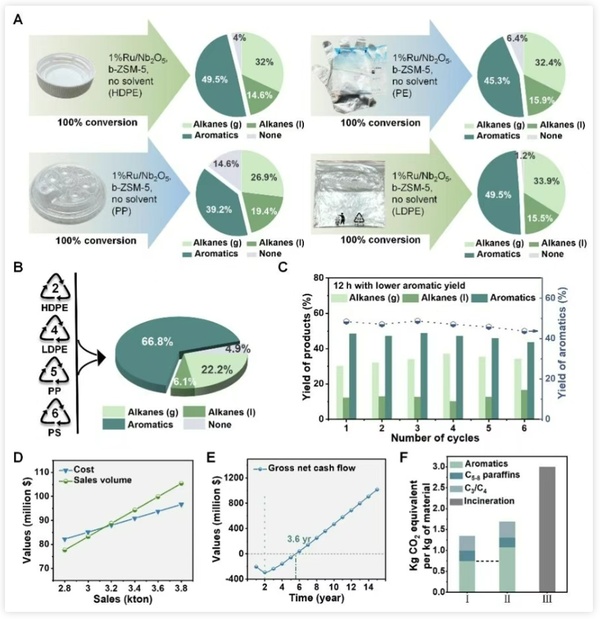
Figure 5. Applicability, adaptability, and techno-economic analysis assessment of the catalytic system.
This study innovatively proposes a new pathway for the low-temperature catalytic conversion of polyolefins into light aromatics. By introducing syngas, the degradation process of waste polyolefins is deeply integrated with the existing petrochemical and coal chemical industry chains. This not only provides a novel perspective for critical C-C coupling industrial processes such as Fischer-Tropsch synthesis but also offers a practical technical solution for addressing plastic waste management and carbon resource recycling.
Wang Yong, Zhejiang University Qiushi Distinguished Professor, Director of the Catalysis Research Institute, recipient of the National Science Fund for Distinguished Young Scholars, and Chief Scientist of the National Key R&D Program. He is the recipient of the China Catalysis Youth Award, the Hou Debang Chemical Science and Technology Innovation Award, and the Green Mountain Science and Technology Award. His research focuses on the fundamental scientific exploration and application of heterogeneous catalysis and energy conversion materials. While deeply engaging in the basic theoretical research of catalysis, he continuously promotes innovation breakthroughs and industrialization practices in green energy technologies. Leading his team, he has laid out an innovation chain around the manufacturing of high-end fine chemicals, deeply integrating advanced catalytic technology, process intensification technology, and the chemical industry. He has developed a series of novel catalytic technologies and green production system solutions with independent intellectual property rights and core competitiveness, achieving industrial production of multiple high-end fine chemicals.JACS, ChemHe has published more than 200 SCI papers in journals, which have been cited over 26,000 times with an h-index of 74; he has been granted more than 50 Chinese invention patents and 5 U.S. patents. As the first contributor, he has won honors such as the China Patent Gold Award, the Special Prize for Technological Invention by the China Petroleum and Chemical Industry Federation, and the First Prizes for Technological Invention and Natural Science in Zhejiang Province.
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

Overseas Highlights: PPG Establishes New Aerospace Coatings Plant in the US, Yizumi Turkey Company Officially Opens! Pepsi Adjusts Plastic Packaging Goals
-
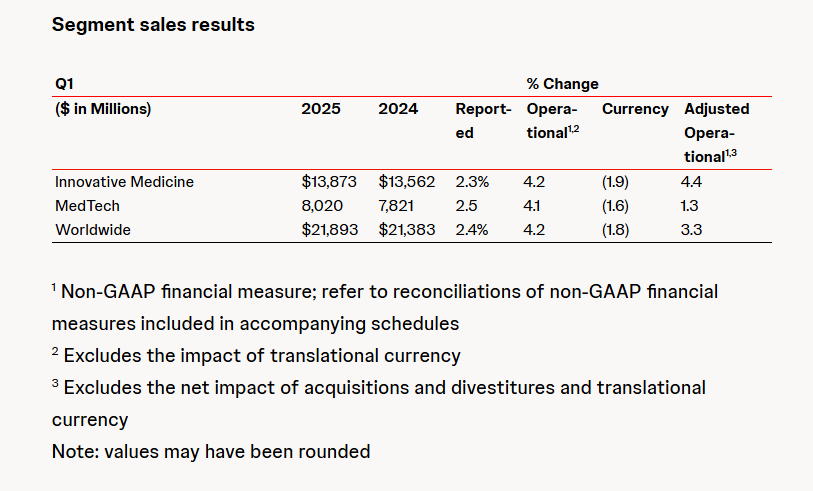
Abbott and Johnson & Johnson: Global Medical Device Giants' Robust Performance and Strategies Amid Tariff Pressures
-

BYD releases 2024 ESG report: Paid taxes of 51 billion yuan, higher than its net profit for the year.
-

Behind pop mart's surging performance: The Plastics Industry Embraces a Revolution of High-End and Green Transformation
-
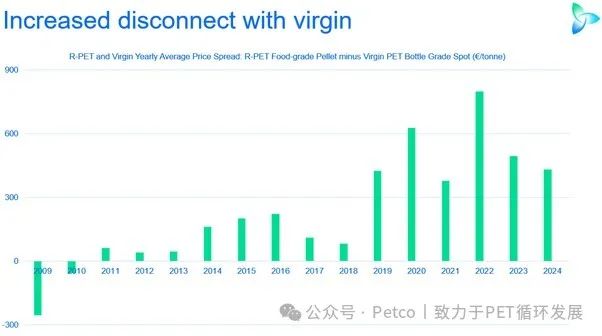
The price difference between recycled and virgin PET has led brands to be cautious in their procurement, even settling for the minimum requirements.



