Effects of Twin-Screw Reaction Extrusion of Long-Chain Branched PP on Wide Molecular Weight Distribution Performance
Polypropylene (PP)As one of the four major general-purpose plastics, it has good overall performance, including excellent heat resistance, chemical resistance, good processability, and cost-effectiveness. Its modification research has always been a hot topic in the industry.
Introducing long-chain branching structures in polymer systems is a common chemical modification strategy. The presence of long-chain branches can cause chain entanglement, which significantly enhances the mechanical properties of polypropylene.
The melt grafting process using a twin-screw extrusion system is not only simple to operate but also offers advantages such as continuous production, high efficiency, and good product uniformity. However, due to the short residence time of materials in the twin-screw extruder, it is difficult to complete the chemical reactions fully, resulting in limited reports on this method. Research institutions usually employ a mixing rotor torque rheometer for the preparation of long-chain polypropylene. Although the reaction time can be controlled and the grafting efficiency is high, the small preparation scale makes it challenging to study the effects of long-chain grafting on the mechanical properties of polypropylene.
Research team of Sinopec Beijing Research Institute of Chemical IndustryMelt graft modification of broad molecular weight distribution polypropylene using a twin-screw reactive extrusion method.The decomposition of peroxide generates free radicals that capture hydrogen atoms on the polymer to form macromolecular radicals. These radicals then react with graft monomers to prepare long-chain branched polypropylene with different monomer addition amounts.Explore the mechanical properties and crystallization properties of modified materials.
Experimental Section
01
Sample preparation
Preparation of Long-Chain Branched Polypropylene in a Twin-Screw Extruder According to the formula in Table 1, weigh the peroxide DMDBH and the monomer PETA, and mix them evenly with PP pellets in a high-speed mixer. Then, add them from the feeding port of the extruder, with a screw speed of 200 r/min. Control the screw torque at 50% by adjusting the feeding amount. The temperature of the extruder screw from the feeding port to the die head is set at 40, 160, 170, 180, 180, 180, 180, and 175°C, and the reaction extrusion for preparing long-chain branched polypropylene is carried out under two sets of conditions at 40, 180, 190, 200, 200, 200, 200, and 195°C.
Mechanical testing sample preparation for injection molding.A series of polypropylene twin-screw reactive extruded modified materials were placed in a drying oven at 80°C for 8 hours, and then molded into standard test specimens using an injection molding machine. The tensile, flexural, and impact specimens were left at room temperature for more than 48 hours to ensure complete cooling and crystallization, and then tested.
02
Performance Testing and Structural Characterization
Sample purification and infrared analysis:Take about 2g of the modified material from the twin-screw extruder and place it in a Soxhlet extractor with xylene as the solvent. Extract for 8 hours at boiling temperature. Quickly pour the resulting solution into acetone to obtain a white precipitate, while the unreacted monomers and oligomers remain dissolved in acetone. Filter to obtain purified polymer powder. Vacuum dry at 80°C for 8 hours. Use a flat plate vulcanizing machine to hot press into films at 200°C and 5MPa for infrared spectroscopy testing.
GPC test:The solvent and mobile phase are both 1,2,4-trichlorobenzene (TCB) (containing 0.025% antioxidant 2,6-di-tert-butyl-p-cresol), tested at 150°C, with a mobile phase flow rate of 1.0 mL/min, using narrow distribution polystyrene standards for universal calibration.
Stretching rheological testing:The specimen dimensions are 40mm×10mm×0.45mm, and the test temperature is 180℃. The sample is supported and kept horizontal by a hot nitrogen stream. The tensile strain rates are 0.3, 1, and 2s^-1, respectively.
DSC Analysis:For each test, approximately 5 mg of sample is used. In a nitrogen atmosphere, initially heat the sample at a rate of 20℃/min to 220℃ and hold for 5 minutes to eliminate thermal history. Then, cool down at a rate of 10℃/min to 50℃ and heat again at a rate of 10℃/min to 220℃ for measurement. Record the change in enthalpy with temperature during crystallization and melting.
Melt mass flow rate testing:According to GB/T 3682.1-2018 test, at a temperature of 230℃ and a load of 2.16kg.
Mechanical performance testing:The tensile fracture stress and fracture strain of the plastic parts were tested according to GB/T 1040—2006, with a tensile speed of 50mm/min. The flexural strength and modulus were tested according to GB/T 9341—2008. Each result is the average of five tests.
Results and Discussion
2.1
FTIR characterization of grafting rate
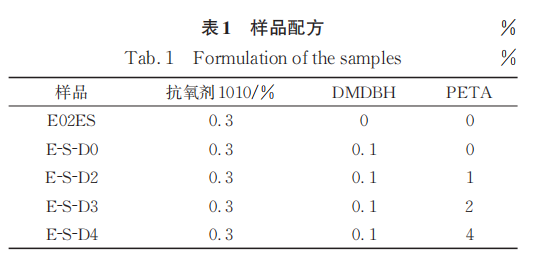
Figure 1 shows the FTIR spectrum and infrared standard curve of the purified sample of E02ES modified material at a reaction temperature of 180°C. For all samples modified with the multifunctional monomer PETA, at 1740cm.-1An absorption peak appears everywhere, which is the absorption peak of the carbonyl group on the ester group in the PETA molecule. The results in Figure 1 indicate that the monomer PETA has been successfully grafted. The carbonyl index (CI) can be calculated according to the formula:
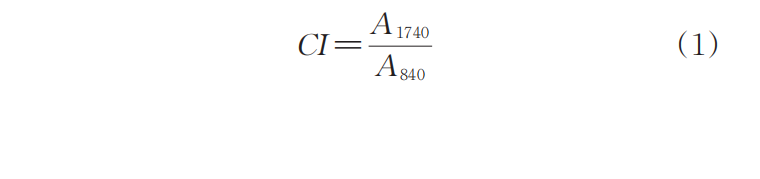
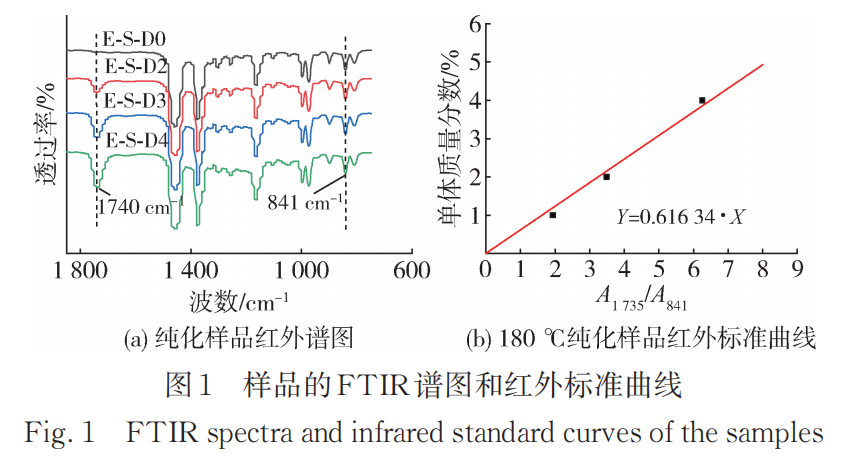
Among them, A1740、A840Wavelength at 1740 cm-1840 cm-1The area of the absorption peak is 841 cm.-1The peak at this position corresponds to the methyl absorption on the polypropylene main chain. CI is only a relative value; to obtain the grafting rate of the monomer, a standard curve needs to be established. The grafting rates of the reaction products with different monomer addition amounts are listed in Table 2.
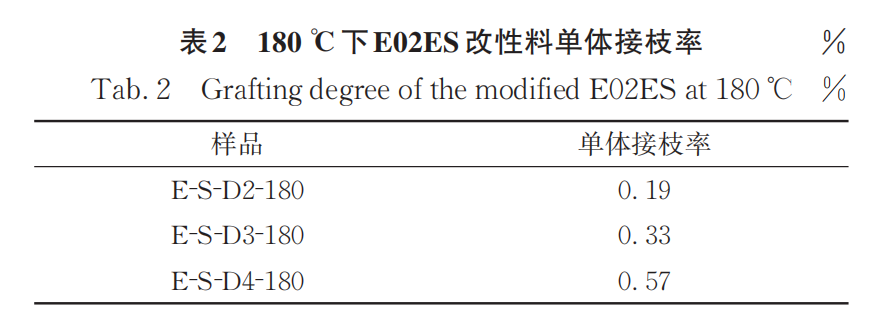
Figure 2 shows the FTIR spectra of purified samples of E-S-D0, 3, and 4 modified materials at a reaction temperature of 200°C, along with the infrared standard curve. Compared to the samples obtained at 180°C, those produced at 200°C exhibit a higher monomer grafting rate (Table 3).
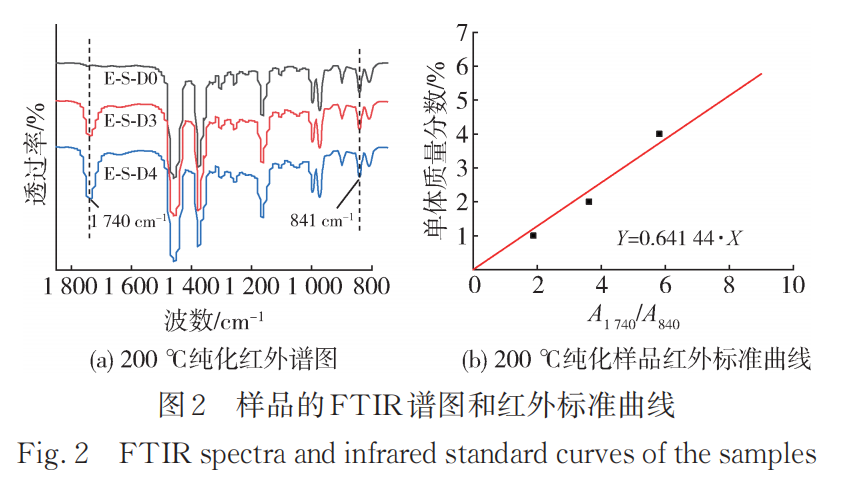
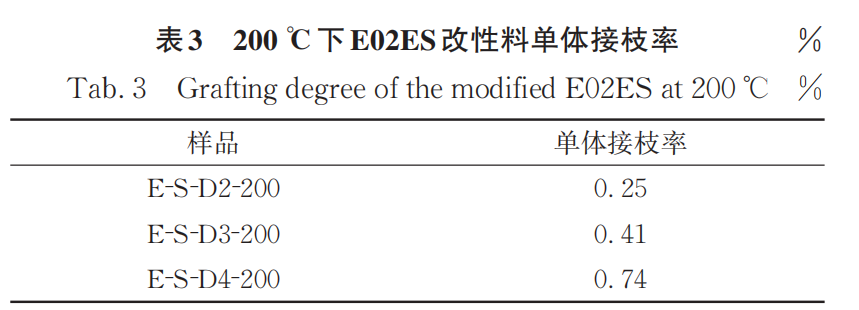
2.2
Molecular weight and its distribution characterization
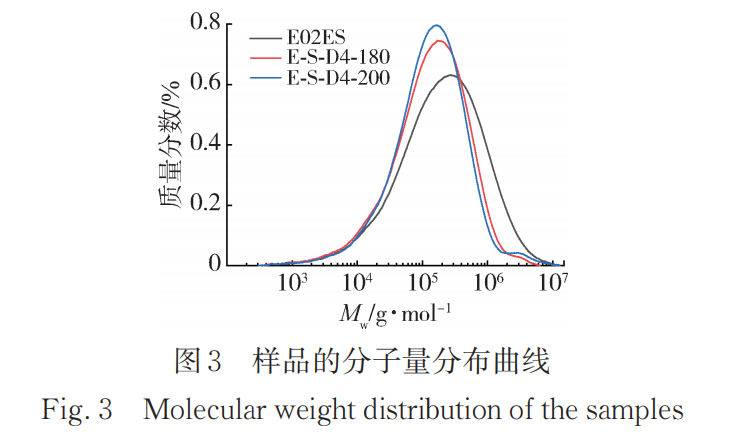
Table 4 lists the molecular weights and polydispersity indexes of the initial E02ES and E-S-D4 at 180°C and 200°C, with the corresponding GPC curves shown in Figure 3. The molecular weight distribution curves of the modified materials at the two different temperatures shift towards lower molecular weights compared to the pure material, and the molecular weight distribution narrows, with the polydispersity index decreasing from around 10 to about 7. The weight-average molecular weight (M)wThe decrease indicates that the addition of peroxide caused a significant degradation reaction, and the modified material has a significantly reduced high molecular weight portion compared to the pure material. This suggests that larger relative molecular weights are more prone to degradation, resulting in a narrower molecular weight distribution.
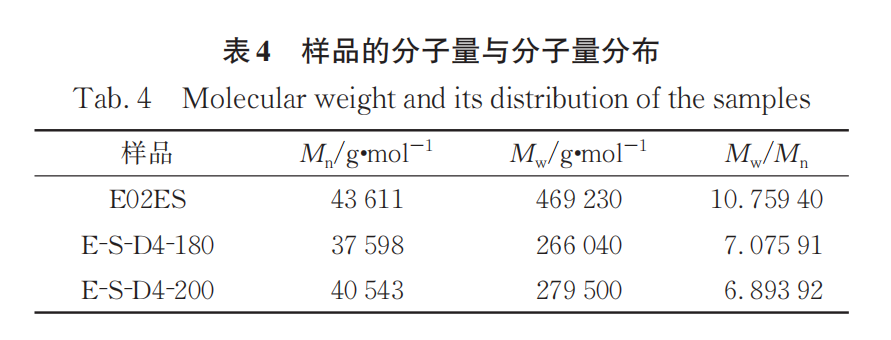
The modified material E-S-D4 at 200℃ compared to the number average molecular weight (M) at the reaction temperature of 180℃.n) and MwThe improvements are slight, indicating that the grafting reaction is more complete at 200℃, allowing the monomer to rapidly react and graft when the peroxide decomposes at a faster rate. Additionally, the modified material at 200℃ shows a small peak at the high molecular weight end of the GPC curve, similar to the small peak in the molecular weight distribution curve of LCBPP obtained by Sugimoto et al. using irradiation methods. It can be concluded that although the increase in temperature may exacerbate degradation, the grafting reaction also improves accordingly.
2.3
Stretching rheological behavior
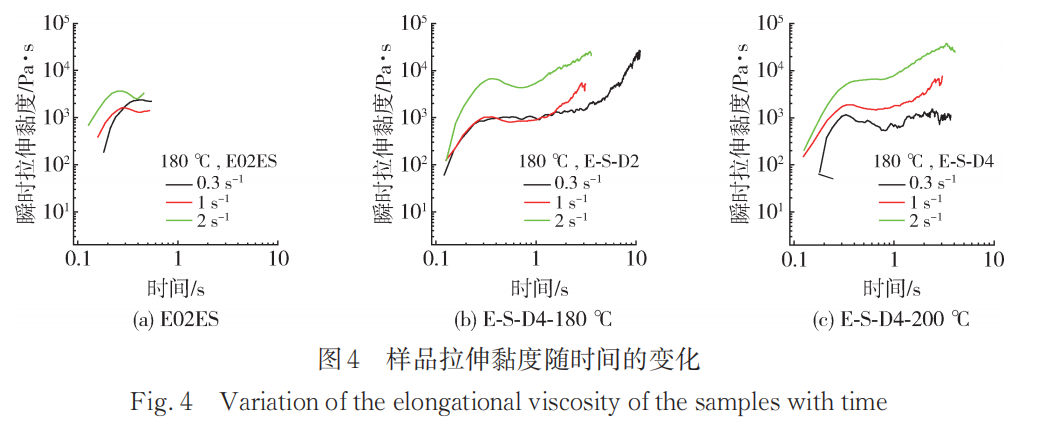
After reactive extrusion of polypropylene, the introduction of long side chains greatly alters its rheological properties. In tensile rheological testing, at higher stretching rates, the tensile viscosity rapidly increases with time, exhibiting strain hardening behavior. This phenomenon occurs because the introduced long side chains entangle, making it difficult for the molecular chains of the material melt to slide under high-temperature stretching conditions, resulting in a significant increase in stress perpendicular to the stretching direction. Therefore, observing the presence of strain hardening in tensile rheological tests can serve as an effective method to characterize the long side chains of polypropylene.
Figure 4 shows the transient stretching viscosity as a function of time at a testing temperature of 180°C for E02ES, E-S-D4-180°C, and E-S-D4-200°C under different stretching rates. It can be seen that the stretching viscosity of the modified materials increases with time at all rates, exhibiting an exponential growth trend. This phenomenon is a clear characteristic of strain hardening, indicating that the samples possess a long-chain structure.
2.4
Effect of Long Branch Modification on the Mechanical Properties of E02ES
2.4.1 Tensile Properties
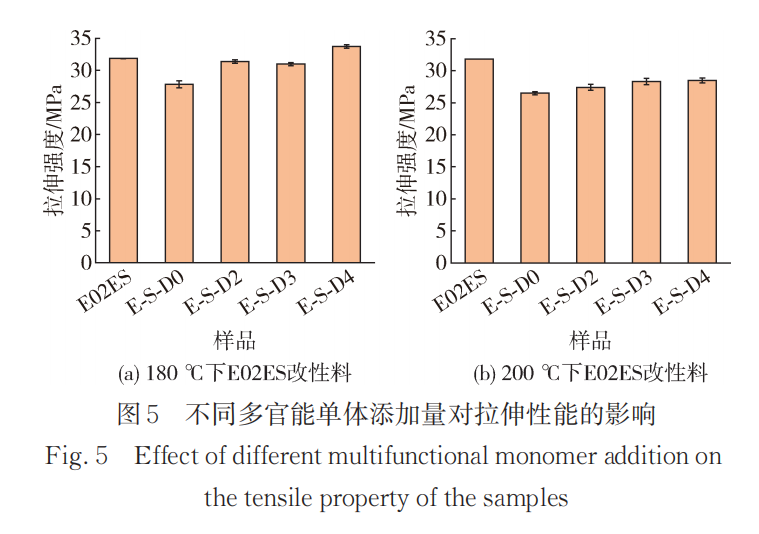
Figure 5 shows the effect of different amounts of multifunctional monomers on tensile properties at reaction temperatures of 180°C and 200°C. At a reaction temperature of 180°C, D0 refers to the reaction mixture with only the addition of peroxide, and the tensile strength decreases significantly. The tensile strengths of E-S-D2 and D3 are similar and close to that of the pure material. This can be explained by the fact that the grafting rates of the monomers in D2 and D3 are similar, resulting in minimal differences in their mechanical performance. The tensile properties of D4 show a slight improvement, likely due to a higher content of long side chains leading to tighter entanglement of molecular chains, which not only compensates for the defects caused by degradation but also enhances the tensile properties compared to the pure material.
At a reaction temperature of 200°C, the degradation reaction intensifies due to the increased temperature, resulting in a noticeable decrease in all modified materials compared to the pure material, by approximately 10%. As the amount of multifunctional monomer added increases, the tensile strength of the modified material improves. It can be concluded that the introduction of long branched chains can improve the tensile properties of the material to a lesser extent.
2.4.2 Bending Performance
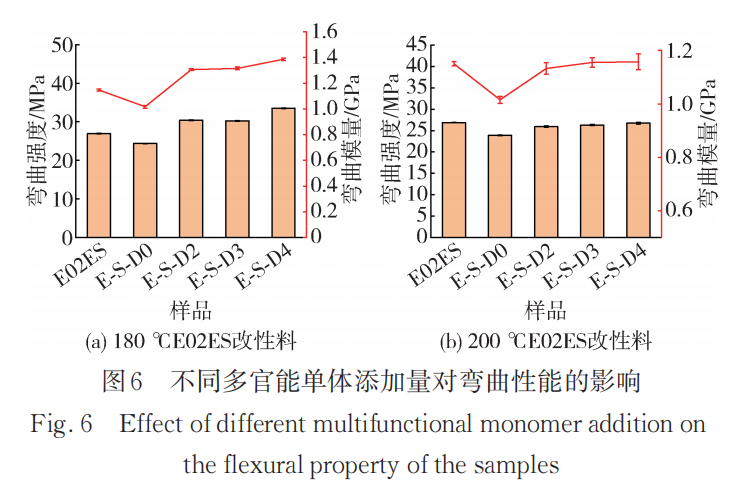
Figure 6 shows the effect of different multifunctional monomer additions on the bending properties at reaction temperatures of 180°C and 200°C. The trend in bending properties is generally consistent with that of tensile properties. At a reaction temperature of 180°C, both the bending strength and bending modulus are significantly improved compared to the pure material. Combined with the results of subsequent melt crystallization characterization work, the presence of long branches effectively increases the crystallization temperature and crystallinity of polypropylene, thereby effectively enhancing the rigidity of the material.
At 200°C, both the bending strength and bending modulus increase slightly with the addition of monomer content. However, the overall bending performance of the modified material at 200°C is lower than that of the modified material at 180°C. This can be understood as, although the modified material at 200°C has a higher crystallization temperature due to the presence of more long side chains, the mechanical properties are more affected by changes in the relative molecular weight. The introduction of long side chains can relatively compensate for the loss of mechanical properties caused by degradation, but due to the low grafting rate of the monomer, significant improvement cannot be achieved.
2.5
Melt Flow Index Test
Tables 5-6 show the melt flow index of the initial E02ES and its modified materials at reaction temperatures of 180°C and 200°C. The melt flow index can reflect the flow properties of thermoplastic polymer materials. At a reaction temperature of 180°C, the relationship of the melt flow index among the samples is D0 > D3 > D2 > D4 > E02ES. It can be seen that the addition of multifunctional monomers can reduce the fluidity of the material. This can be considered as the result of two factors acting simultaneously: first, the introduction of long branched chains increases physical entanglement, which strengthens intermolecular forces and weakens fluidity; second, the multifunctional monomers have the ability to capture free radicals, leading to a relatively reduced proportion of degradation reactions. Among them, the melt flow index of E-S-D2 is greater than that of E-S-D3, which is still consistent with the infrared spectroscopy results.
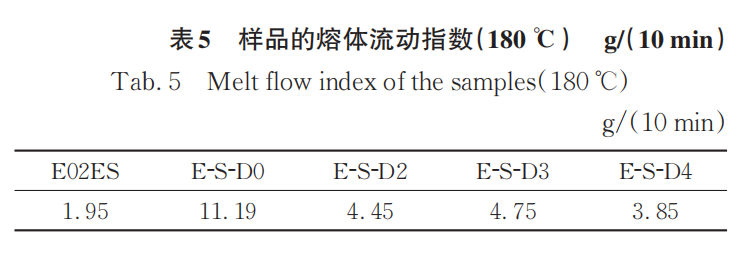
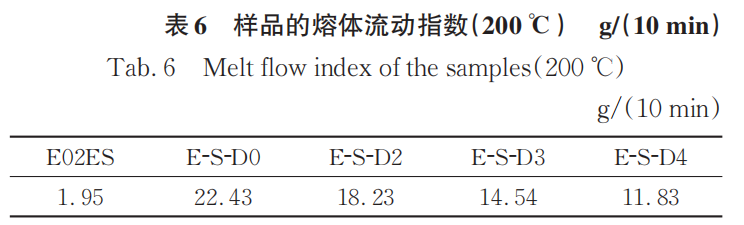
When the reaction temperature is 200°C, the melt flow index of the modified material significantly increases due to the degradation reaction. Additionally, as the amount of monomer added increases, the melt flow index correspondingly decreases, further proving that the grafting reaction is more complete at 200°C.
2.6
Melting crystallization behavior
Figure 7 shows the DSC heating and cooling curves of the initial E02ES and its modified materials at 180°C, with the corresponding crystallization and melting parameters listed in Table 7. As can be seen from Table 7, the crystallization temperature increased by more than 5°C after long chain branching compared to the initial E02ES, and the crystallization temperature slightly increased with the addition of the monomer. Short chain branching PP can also cause changes in the crystallization temperature, although the extent is very limited. It is evident that the significant increase in crystallization temperature mainly comes from the contribution of long chain branches, which can act as "heterogeneous nucleation sites" in the system, advancing the nucleation time. Moreover, Ono et al. reported that when molecular chains are entangled, the crystallization growth rate is independent of molecular weight, thus eliminating the possibility of crystallization rate changes due to degradation.
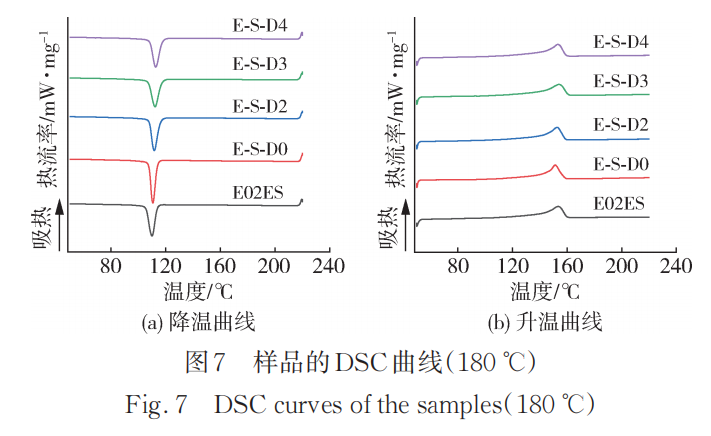
The crystallinity of the modified crystallization material decreases compared to the initial E02ES due to the disruption of chain regularity after grafting long side chains. Although the nucleation rate increases, the rate of chain segment incorporation into the lattice slows down.
Figure 8 shows the heating and cooling curves of the initial E02ES and its modified materials at 200℃, with the corresponding crystallization and melting parameters listed in Table 8. Compared to the data at 180℃, the crystallization temperature of the modified materials at 200℃ further increased, once again verifying the conclusions from infrared spectroscopy. Among them, the crystallization temperature of E-S-D0 is also higher than that of the initial E02ES, due to the degradation reaction enhancing the mobility of the molecular chains, allowing them to more quickly arrange into the crystal lattice.
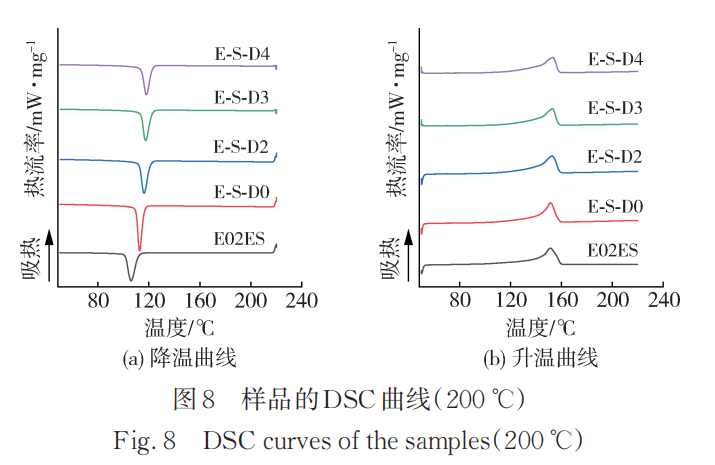
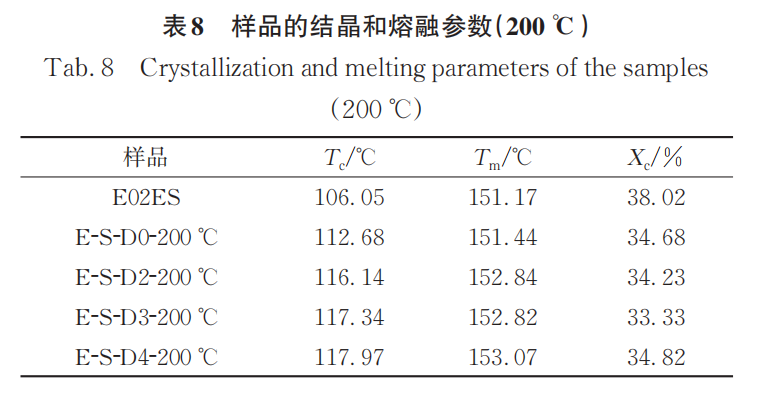
The physical entanglement formed by long branched chains or the improved crystallization performance can effectively enhance the physical properties of polypropylene.
The introduction of long branches can serve as nucleation points, leading to an increase in crystallization rate, while also causing a decrease in crystallinity.
(3) The product obtained by reactive extrusion at 200 ℃ has a higher monomer grafting rate, and the crystallization temperature increases significantly. However, the mechanical properties of the product also decrease noticeably due to the increased proportion of degradation reactions. Therefore, the reactive extrusion temperature needs to be determined according to the specific requirements of the final product.
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

India's Q3 Smartphone Shipments Rise 3%; Japanese Mold Factory Closures Surge; Mercedes-Benz Cuts 4,000 Jobs
-

Ascend's Restructuring Plan Approved! Jwell Launches Global Acceleration Plan; Nexperia Chip Crisis Threatens Global Auto Production
-

Dow To Restart Pe Units 5 And 7 This Week, Recovery Date For Unit 6 Remains Undetermined In The United States (US)
-

Key Players: The 10 Most Critical Publicly Listed Companies in Solid-State Battery Raw Materials
-

The Roller-Coaster Behind Sanhua Intelligent Controls' Stock Price: What Are the Advantages of Automotive Thermal Management Companies Crossing Into Humanoid Robots?






