Factors to Consider When Choosing the Right Flame Retardant
This article will focus on the selection logic of flame retardants, providing you with a clear and practical screening guide from multiple dimensions such as application requirements, performance balance, cost control, and processing adaptability.

The choice of flame retardant depends on:
Application scenario type.
Specific flame retardant standards to be met
Relevant regulations that need to be complied with
When selecting the best flame retardant system for a specific application, many other issues must also be considered.
Selection Criteria for Brominated Flame Retardants
Factors Influencing the Selection of Brominated Flame Retardants
Types and Content of Bromine
For the selected brominated flame retardants to be effective, they need to decompose during the combustion of the polymer while remaining stable during the polymer processing. This requirement determines the type of bromine in the flame retardant. Additionally, the flame retardant must contain sufficient bromine content to achieve the desired flame retardant performance, and its addition should not adversely affect the physical properties of the material or the system cost due to excessive amounts.
Thermal stability
The selected brominated flame retardants must remain stable during blending and injection molding processes. If decomposition occurs during these processes, it may lead to material discoloration, polymer degradation, and equipment corrosion. Therefore, it is essential to choose suitable flame retardants.And match with the required heat stabilizers and synergists.Crucial.
Aging characteristics
Resin systems may need to withstand various factors that lead to premature degradation and discoloration of their performance. When selecting the most suitable flame retardant and required stabilizers for a specific system, factors such as UV resistance, thermal stability, and migration must be comprehensively considered.
Processing characteristics
According to the different processing temperatures, some flame retardants can be melt-blended, while others are used as fillers—this difference will affect the processing process and the physical properties of the final product.
Standards to be met
The selection of flame retardants largely depends on the chosen resin system and the standards that need to be met.
Usage cost
The total cost of the flame retardant system needs to be considered, which depends not only on the price of the brominated flame retardants themselves but also on the amount required for addition. Additionally, it relies on the cost of other additives needed to create a usable system in conjunction with the brominated flame retardants.
Environmental considerations
The use of brominated flame retardants poses specific environmental restrictions, with one of the core issues being the reduction of toxic hazards throughout the entire production process (from manufacturing and end use to disposal).
Resistance to spray
Blooming refers to the phenomenon where flame retardants slowly migrate to the surface of plastics, resulting in a hazy appearance on the material, often resembling a bronze-like color. This phenomenon is particularly undesirable for components such as casings and housings that also have aesthetic functions. Therefore, in certain applications, anti-blooming properties are a key consideration.
The severity of the frosting phenomenon usually depends on the compatibility of the flame retardant with polymer additives and the molecular weight of the flame retardant.The better the compatibility and the higher the molecular weight, the less pronounced the frosting phenomenon.。
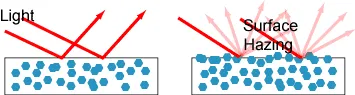
(Left: no frosting, Right: surface fogging)
UV stability
In many applications, flame-retardant resins may need to withstand various environmental conditions, which can easily lead to premature deterioration and discoloration of material properties. Therefore, for applications requiring UV resistance (such as outdoor applications), selecting the appropriate brominated flame retardant is crucial.
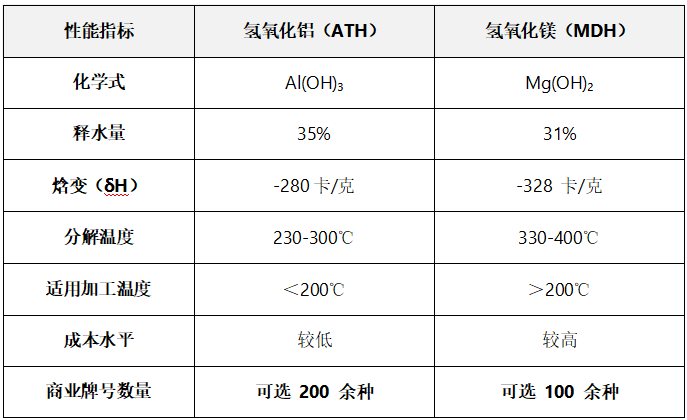
Aluminum hydroxide (ATH) and magnesium hydroxide (MDH)
Selection Criteria
When selecting aluminum hydroxide (ATH) or magnesium hydroxide (MDH) products for flame retardant formulations, key material parameters should be carefully considered.
Median particle size
Particle size distribution
Specific surface area
Particle morphology
Surface chemical properties
Color
The product characteristics of these (basic materials) willDirectly affects the blending process and the final performance of the composite.。
Physical performance comparison
Thermal Stability Comparison
The following figure compares the thermal decomposition characteristics of aluminum hydroxide (ATH) and magnesium hydroxide (MDH).
Aluminum hydroxide begins to decompose at approximately 220°C (428°F).
Magnesium hydroxide decomposes at approximately 330°C (626°F).
Therefore, magnesium hydroxide has higher thermal stability, providing a wider temperature window for blending processing. Aluminum hydroxide is suitable for thermosetting plastics processed at temperatures typically below 200°C, as well as certain PVC and polyolefin-based plastic compounds.
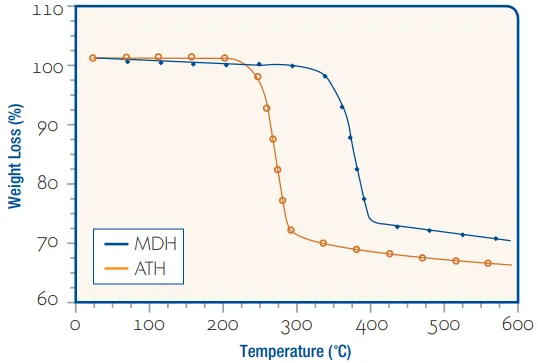
Source: Huber Engineered Materials
When preparing plastic composites that need to be processed at temperatures close to or above 220°C (428°F), magnesium hydroxide is preferred. For example, polypropylene and engineering thermoplastic materials.For low melting point thermoplastics or elastomers, using magnesium hydroxide can also achieve higher processing temperatures and higher blending yields.。
Aluminum hydroxide and magnesium hydroxide both release crystallization water when decomposed by heat: on one hand, they cool the polymer matrix, and on the other hand, they dilute the smoke generated during combustion (the water release of aluminum hydroxide is approximately 35% of its weight, while that of magnesium hydroxide is about 31%). At the same time, the endothermic decomposition process of both can absorb a large amount of heat, further suppressing the combustion reaction. When calculated per unit weight, the heat absorption of magnesium hydroxide (328 cal/g) is higher than that of aluminum hydroxide (280 cal/g).The flame retardant efficiency is better in high-temperature scenarios.。
Filler addition amount
To achieve the desired flame retardant effect, aluminum hydroxide (ATH) and magnesium hydroxide (MDH) typically require an addition level of 150 phr (approximately 60% by weight). However, high addition levels can easily lead to a decrease in material processability, while also impairing mechanical properties (such as tensile strength and impact strength) and physical properties (such as toughness and weather resistance).
Despite the lower unit price of hydroxide fillers compared to most other types of flame retardants, the overall cost advantage due to high addition levels is not significant. To address this issue, the industry typically adopts the following solutions:
Cooperating with synergistic additivesAdding synergistic agents to hydrated metal hydroxide fillers can reduce the amount of filler added without compromising flame retardant properties, alleviating the decline in processability and mechanical properties.
Surface modification treatmentThe surface treatment of hydroxide fillers using organosilanes, zirconate esters, or titanate esters can enhance their compatibility with polymer matrices, thereby increasing their utilization efficiency.
Compound useThere is a synergistic effect between aluminum hydroxide and magnesium hydroxide—when the total addition amount remains constant, their combination can improve flame retardant performance; or when the flame retardant performance remains constant, it can reduce the total addition amount. Additionally, compounding metal hydroxides with ATH/MDH can further enhance the flame retardant effect.
Advantages of applying composite metal hydroxides
In the extrusion test, replacing pure MDH with a mixture of MDH/ATH in different ratios can achieve the following processing optimization:
Mold pressure reduced by 15% to 20%; torque reduced by 16% to 21%, resulting in decreased energy consumption.
Using a mixture of aluminum hydroxide/magnesium hydroxide and synergistic mineral flame retardants can reduce costs. The price order of various related additives from high to low is: polypropylene > MDH > ATH > ordinary white fillers.
Utilizing this price difference and synergistic effects, a good balance can be achieved between flame retardant performance, physical and mechanical properties, and cost, typically resulting in cost savings of 25%-30%.
The details are shown in the figure below: The caption indicates MDH control group, MDH/ATH mixture, MDH/ATH/kaolin mixture, MDH/ATH/calcium carbonate mixture; the horizontal axis is the cost index, ranging from 0-150.
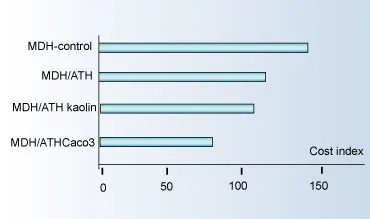
(Cost-Effectiveness Comparison of Composite Metal Hydroxides)
At this point, you can preliminarily determine the type of flame retardant according to the application scenario, but further verification of the compatibility between the flame retardant and the plastic matrix in the formulation is still needed.The final selection must simultaneously meet the processing characteristics, performance requirements, and flame retardant requirements of the polymer.。
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

List Released! Mexico Announces 50% Tariff On 1,371 China Product Categories
-

Dow, Wanhua, Huntsman Intensively Raise Prices! Who Controls the Global MDI Prices?
-

Clariant Unveils Cost-Cutting Plan Details, Plans to Shut Down Multiple Plants
-

New Breakthrough in Domestic Adiponitrile! Observing the Rise of China's Nylon Industry Chain from Tianchen Qixiang's Production
-

Nissan Cuts Production of New Leaf EV in Half Due to Battery Shortage






