Study on the performance of modified polyurethane coatings in high salinity environments
Keyword
1. Introduction
Concrete structures are widely used in dams, channels, and retaining walls, and their long-term durability is crucial for the safety and economic benefits of hydraulic engineering.[1]However, corrosive media such as chloride ions in high-salt environments (such as coastal areas and saline areas) can accelerate the corrosion of steel reinforcement in concrete.[2]This leads to deterioration and cracking of the structure, weakening the durability and safety of the concrete.[3]Therefore, developing efficient protective measures to slow down the corrosion process and enhance the durability of concrete structures has become an urgent issue to be addressed.
Polyurethane coatings are widely used in the protection of concrete structures due to their good adhesion, excellent waterproof performance, and resistance to chemical corrosion.[4]However, traditional polyurethane coatings have certain limitations in high-salt environments, such as reduced coating density and decreased salt corrosion resistance when exposed to corrosive environments for extended periods.[5]In recent years, researchers have been committed to modifying polyurethane coatings with nanomaterials to enhance their density, mechanical properties, and corrosion resistance, thereby affecting the protective effect.[6]Graphene oxide (GO) in nanomaterials, due to its high specific surface area and abundant oxygen-containing functional groups, demonstrates excellent modification potential. GO can not only enhance the barrier properties of the polyurethane matrix but also improve the mechanical strength of the coating by forming strong hydrogen bonds.[7]However, research on how to effectively and uniformly disperse GO and optimize its performance in coatings is still insufficient.
This study aims to design experiments with different amounts of GO added to investigate its effects on the impermeability, salt corrosion resistance, and mechanical properties of polyurethane coatings, in order to clarify the modification effects of GO and determine the optimal amount to be added.
2 Materials and Methods
2.1 Materials and Instruments
Materials: polyether polyol (PU), isocyanate, GO, silane coupling agent, dibutyltin dilaurate, polyether-modified polysiloxane dispersant, acrylate-modified polysiloxane leveling agent, dimethylformamide, sodium chloride, etc.
Instruments: Ultrasonic disperser, magnetic stirrer, oven, scanning electron microscope (SEM), Fourier-transform infrared spectrometer (FTIR), permeability tester, chloride ion permeability testing equipment, hardness tester, pull-off tester, freezer, electronic balance, film applicator, etc.
2.2 Preparation of Modified Polyurethane Coating
2.2.1 Surface Modification Treatment of Nanomaterials
(1) Weigh 5 g of GO and place it into a 250 mL beaker.
(2) Add 100 mL of deionized water to the beaker and disperse the GO in the water.
(3) Place the beaker in an ultrasonic disperser and treat it ultrasonically for 30 minutes. (4) Add 0.1 g of γ-aminopropyltriethoxysilane, which is 2% of the mass of GO, slowly into the GO dispersion.
(5) Continue to ultrasonically treat the mixed solution for 1 hour, then transfer the dispersion to a drying oven set at a temperature of 60°C and dry for 12 hours to obtain the modified GO powder.
2.2.2 Paint Formulation
(1) Weigh 100 g of polyether polyol.
(2) Add 5 groups with addition ratios of 0.5%, 1.0%, 1.5%, 2.0%, and 3.0% by mass. For example, in the 1.0% GO group, 1 g of modified GO needs to be weighed.
(3) Add modified GO into polyol, stir with a magnetic stirrer for 20 minutes, and then perform ultrasonic dispersion for 30 minutes.
(4) Weigh 30 g of isocyanate and slowly add it to the polyol mixture, stirring continuously for 10 minutes.
(5) Add 0.5 g of dibutyltin dilaurate, 1.0 g of polyether-modified polysiloxane, and 0.5 g of acrylate-modified polysiloxane, then stir for 10 minutes to obtain the modified polyurethane coating solution.
Coating and Curing
(1) Clean the concrete specimens thoroughly and prepare specimens with dimensions of 10 cm × 10 cm × 2 cm. (2) Use a film applicator to evenly coat the surface of the concrete specimens with a modified polyurethane layer, maintaining a coating thickness of approximately 200 μm. (3) Place the coated specimens in a room temperature environment to cure for 24 hours, then continue drying them in a 60°C oven for an additional 12 hours.
2.3 Experimental Method
(1) Anti-seepage performance. ① Cut the cured coating concrete specimen into standard dimensions of 10 cm×10 cm×2 cm; ② Install the specimen on the permeability tester, set the water pressure to 0.1 MPa and keep it constant, conduct a 24-hour permeability test, and record the changes in permeability every 2 hours.
(2) Chloride ion permeability performance. ① Soak the specimen in a 3.5% NaCl solution for 24 hours, then place the specimen in the test chamber of the chloride ion permeability testing equipment, injecting 3.5% NaCl solution on one side and deionized water on the other side; ② Apply a voltage of 60 V, maintain for 6 hours, and record the amount of charge passed through.
(3) Salt freeze-thaw cycle test. ① Prepare a 5% NaCl solution by mass concentration and immerse the coated specimen in the solution for 24 hours, then freeze at -20°C for 24 hours, and thaw at 20°C for 24 hours to complete one salt freeze-thaw cycle; ② Weigh the specimen after every 10 cycles; ③ Repeat the salt freeze-thaw cycle 50 times.
(4) Mechanical properties. Testing is conducted using a pull-off tester, applying tensile force until the coating detaches from the substrate, and recording the adhesion strength. Measure the hardness at 5 locations on each specimen and calculate the average value.
3 Results and Analysis
3.1 Microstructure
Figure 1 shows the SEM characterization of modified polyurethane coatings with different amounts of GO added. Figures 1 (a) to (f) represent the SEM characterization results of polyurethane coatings with unmodified, 0.5%, 1.0%, 1.5%, 2.0%, and 3.0% GO additions, respectively.
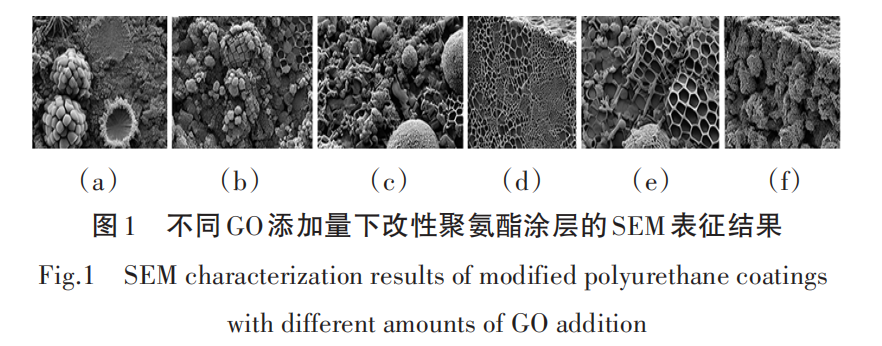
From Figure 1, it can be seen that the surface of the unmodified polyurethane coating appears relatively rough, with noticeable pores and uneven areas. With a 0.5% GO addition, the pores on the surface of the coating are somewhat reduced. As the GO addition increases to 1.5%, the density of the coating further improves, exhibiting a more uniform and smooth surface structure. This indicates that at this point, the dispersion of GO in the matrix is better, effectively filling the pores in the matrix.[8]When the GO addition reaches 2.0% and 3.0%, although the coating's density remains good, GO agglomeration can be observed in local areas. This indicates that a high addition of GO is difficult to continue to uniformly disperse in the polyurethane matrix, which may lead to the protective performance of the coating becoming flat or being affected.
3.2 Infrared Analysis
Figure 2 shows the FTIR analysis results of modified polyurethane coatings with different GO additions.
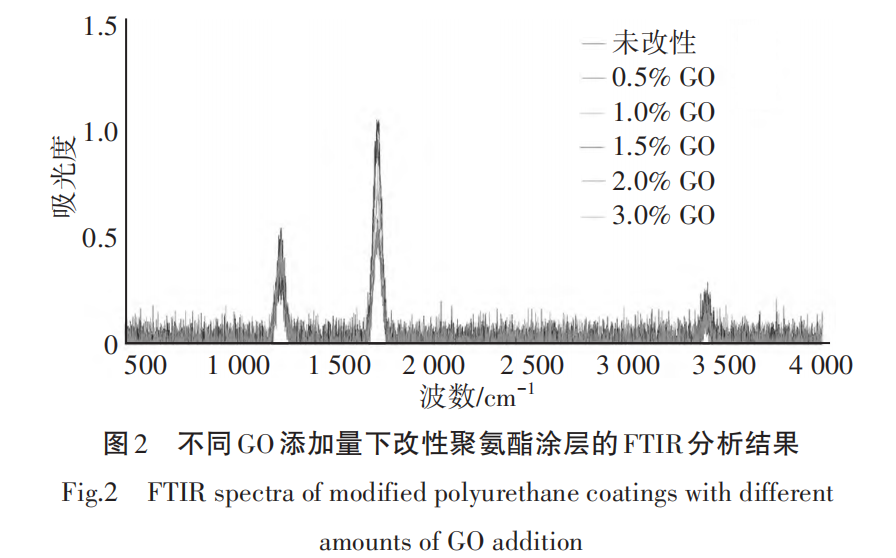
From Figure 2, it can be seen that in the unmodified polyurethane coating, 1,700 cm.-1There is a distinct C=O stretching vibration peak, indicating the presence of a significant amount of ester groups in the polyurethane matrix. As the amount of GO added increases, the C=O stretching vibration peak gradually weakens, especially at 1.5% GO, where the weakening is most significant. This suggests that the modified coating contains a reduced amount of ester groups, which may be related to the formation of a cross-linked structure by GO in the matrix.[9] . Meanwhile, 1,200 cm-1The nearby C—O stretching vibration peak gradually strengthens, indicating that the addition of GO promotes the formation of more chemical bonds within the coating, thereby enhancing the crosslinking density.
In 3,200~3,500 cm-1Within the range, the unmodified polyurethane coating exhibits a strong —OH stretching vibration peak. In the GO modified polyurethane coating, as the amount of GO increases, the —OH absorption peak gradually weakens, indicating that the addition of GO significantly reduces the free hydroxyl groups in the matrix and enhances the cross-linking degree of the coating.
3.3 Material Properties
3.3.1 Impermeability Performance
The test results of the impermeability of coating materials with different amounts of GO added are shown in Table 1.
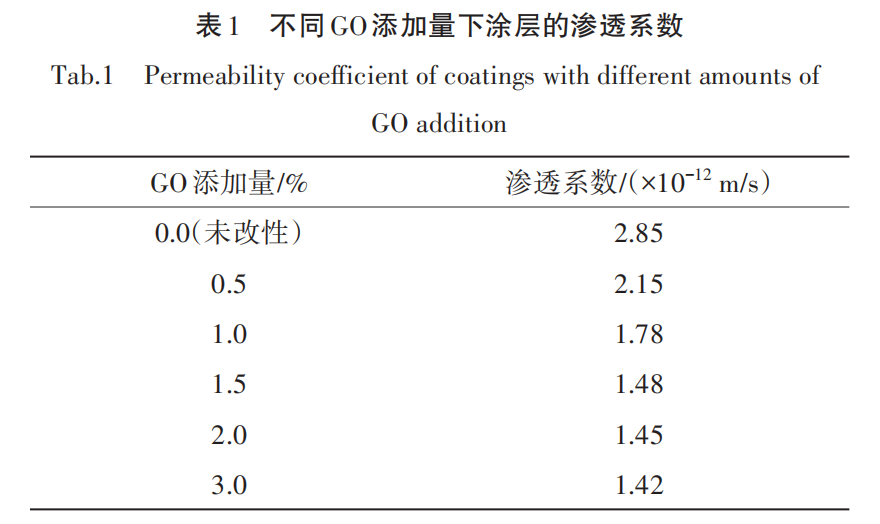
From Table 1, it can be seen that with the increase in the amount of GO added, the impermeability of the modified polyurethane coating is significantly enhanced. This is mainly attributed to the good dispersibility of GO and its interaction with the matrix. The unmodified polyurethane coating has a high permeability coefficient of 2.85×10^-12 m/s, indicating its weak performance in preventing moisture penetration. As the amount of GO added increases, the permeability coefficient gradually decreases, particularly at a 1.5% GO addition, where the permeability coefficient reduces to 1.48×10^-12 m/s, showing a significant enhancement in impermeability. At this concentration, it ensures good dispersibility while greatly improving impermeability. At 2.0% and 3.0% GO additions, although the permeability coefficient continues to slightly decrease, the reduction is no longer significant, as excessive GO cannot be completely and uniformly dispersed within the polyurethane matrix.[10]Leading to local agglomeration and affecting the uniformity of the coating.
3.3.2 Salt Corrosion Resistance Performance
The experimental results of chloride ion permeability and mass loss rate after salt freeze cycles of modified polyurethane coatings with different GO additions are shown in Table 2.
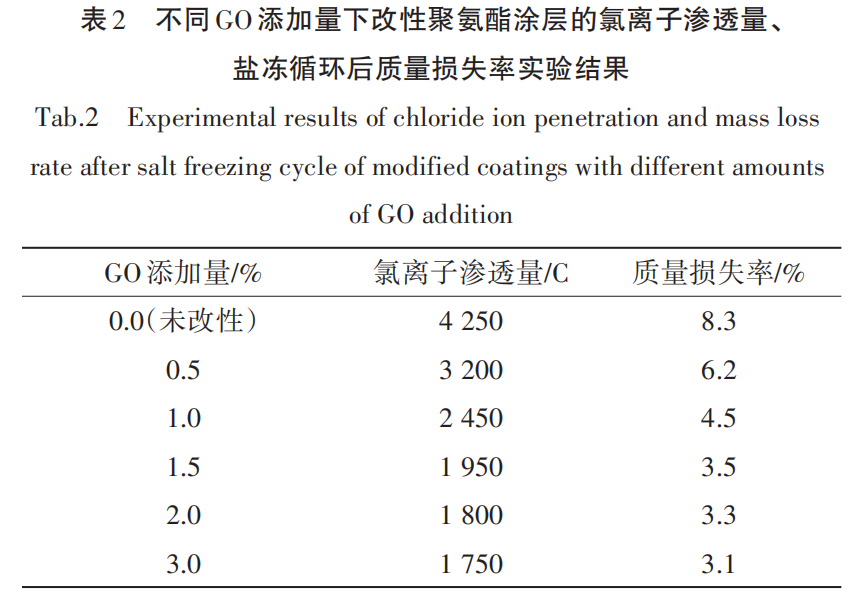
(1) Chloride ion permeability. According to Table 2, the chloride ion permeability of the unmodified polyurethane coating is 4,250 C, indicating a significant deficiency in preventing chloride ion penetration.[11]As the amount of GO added increases, the chloride ion penetration significantly decreases. When the GO addition reaches 1.5%, the chloride ion penetration reduces to 1,950 C, indicating a substantial improvement in the coating's resistance to chloride ion penetration. At GO addition levels of 2.0% and 3.0%, the penetration further decreases, but the rate of decrease tends to level off, reaching 1,800 C and 1,750 C respectively.
(2) Salt freeze-thaw cycle test. As shown in Table 2, the unmodified polyurethane coating exhibits a mass loss rate of 8.3% after 50 salt freeze-thaw cycles, indicating relatively low resistance to salt freeze-thaw conditions. With the increase in GO content, the mass loss rate of the coating gradually decreases. At a 1.5% GO content, the mass loss rate drops to 3.5%. When the GO content is further increased to 2.0% and 3.0%, the reduction is no longer significant, indicating that the improvement in salt freeze-thaw resistance tends to saturate when the GO content exceeds a certain range.[12]This may be related to the agglomeration of GO in the matrix, where an excessive amount of GO is not easily dispersed evenly, leading to local agglomeration that affects the structural uniformity of the coating, thereby limiting further improvement in coating performance.
Mechanical Properties and Durability
The test results of the mechanical properties (adhesion and hardness) of polyurethane coatings with different amounts of GO added, as well as the hardness retention under UV aging and damp heat aging conditions, are shown in Table 3.
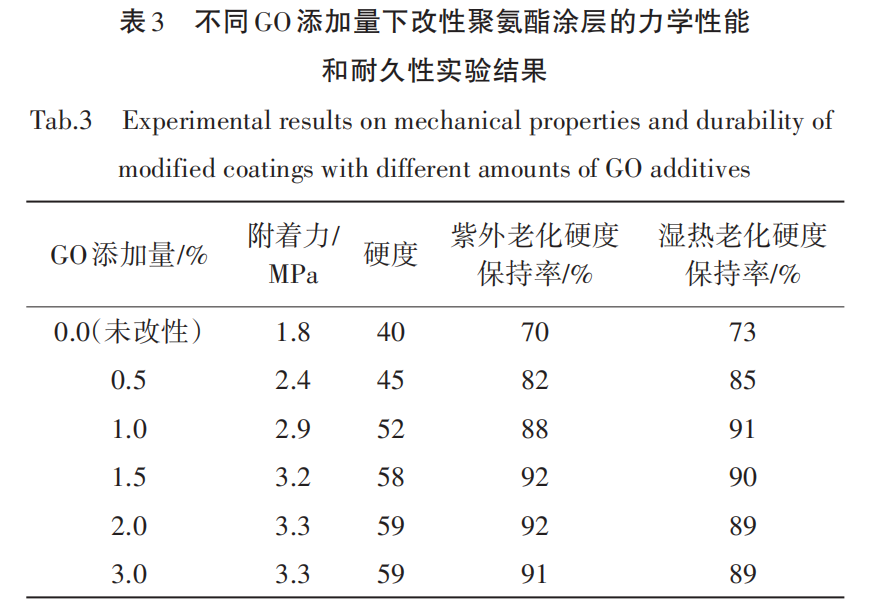
(1) Mechanical Properties. As shown in Table 3, the adhesion of the unmodified polyurethane coating is 1.8 MPa, and the hardness is 40, indicating that the bonding strength between the coating and the concrete substrate is relatively low, and the hardness has not reached the ideal value. With the increase in GO content, the adhesion of the coating gradually improves. At a GO content of 1.5%, the adhesion reaches 3.2 MPa, and the hardness increases to 58. However, when the content increases to 2.0% and 3.0%, the increments in adhesion and hardness begin to diminish, reaching 3.3 MPa and 59, respectively, suggesting that an excessive amount of GO may lead to uneven dispersion within the substrate, thus affecting further improvements in mechanical properties.
(2) Durability. As shown in Table 3, the unmodified polyurethane coating experienced a 30% decrease in hardness after 500 hours of UV aging and a 27% decrease after damp heat aging, indicating its poor durability under long-term aging conditions. In contrast, the GO-modified polyurethane coating exhibited optimal durability when 1.5% GO was added, with only an 8% decrease in hardness after UV aging and a 10% decrease after damp heat aging. This indicates that the addition of GO significantly enhanced the coating's weather resistance and moisture resistance, improving its adaptability to harsh environments. However, when the GO content was increased to 2.0% and 3.0%, the improvement in hardness retention tended to level off, which might also be due to the local agglomeration caused by excessive GO addition, affecting the uniformity and durability of the coating.
3.3.4 Comprehensive Performance
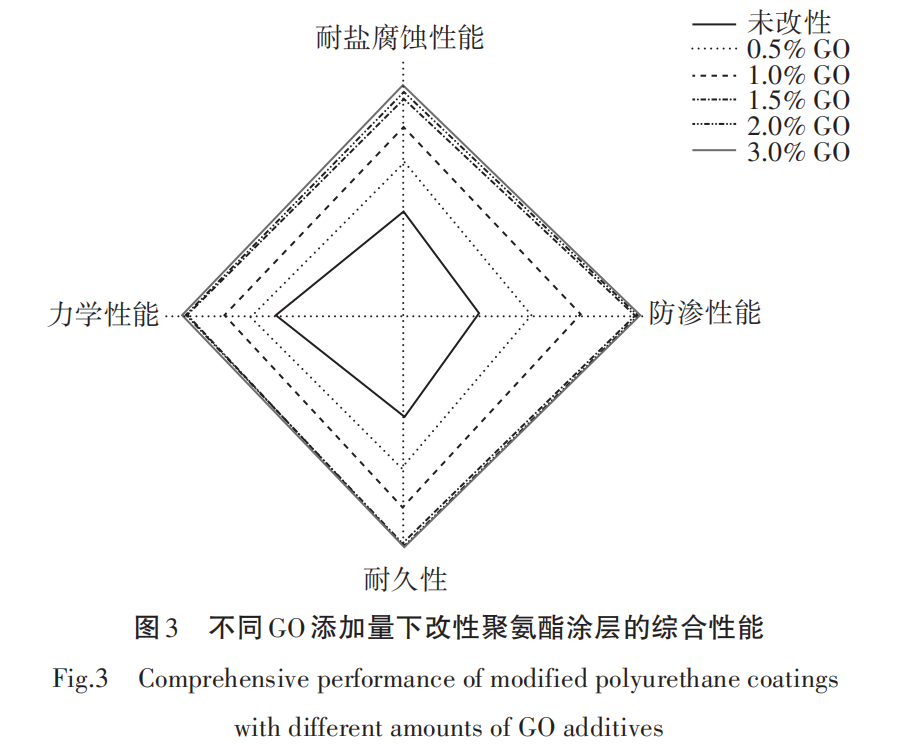
Figure 3 shows the radar chart of the comprehensive performance of modified polyurethane coatings. As can be seen from Figure 3, the performance indicators of unmodified polyurethane coatings are relatively low. However, with the gradual increase in GO content, the modified polyurethane coatings exhibit significant improvements in various performance aspects, especially at a 1.5% GO content, showing remarkable comprehensive advantages in terms of impermeability, salt corrosion resistance, mechanical properties, and durability. When the GO content increases to 2.0% and 3.0%, the rate of performance improvement significantly slows down, which may be related to the limited dispersion and agglomeration of GO in the matrix. Local GO agglomeration may weaken its reinforcing effect on the matrix.
Conclusion
At a 1.5% addition of GO, the anti-permeation, salt corrosion resistance, and mechanical properties of the polyurethane coating achieve optimal results. The permeability coefficient decreases by approximately 47.8%, chloride ion penetration is reduced by 35%, the mass loss rate in the salt freeze-thaw cycle test drops to 3.5%, adhesion improves by 68%, and hardness is significantly increased. SEM analysis indicates that the coating surface of the 1.5% GO group is denser, with noticeably fewer pores, while FTIR shows enhanced cross-linking structure. However, as the GO addition continues to increase to 2% and 3%, the improvement in coating performance tends to plateau or even slightly decline, possibly due to GO agglomeration, which reduces dispersion uniformity and structural density.
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

List Released! Mexico Announces 50% Tariff On 1,371 China Product Categories
-

Dow, Wanhua, Huntsman Intensively Raise Prices! Who Controls the Global MDI Prices?
-

Clariant Unveils Cost-Cutting Plan Details, Plans to Shut Down Multiple Plants
-

New Breakthrough in Domestic Adiponitrile! Observing the Rise of China's Nylon Industry Chain from Tianchen Qixiang's Production
-

Nissan Cuts Production of New Leaf EV in Half Due to Battery Shortage






