3 Dead, 167 Seriously Injured! Boston Scientific's Star Product Release Alert
On January 16, 2026, the U.S. Food and Drug Administration (FDA) announced that Boston Scientific has issued a notification to customers advising them to: Immediately discontinue use and return specific lots of AXIOS™ Stent and Electrocautery Enhanced Delivery System.
This product is currentlyAmerica's only A stent system approved for endoscopic ultrasound-guided drainage of symptomatic pancreatic pseudocysts and walled-off necrosis, with some models also suitable for gallbladder drainage in patients with acute cholecystitis.

The system integrates an electrocautery catheter with a stent, aiming for a single, no-instrument-exchange surgical procedure.
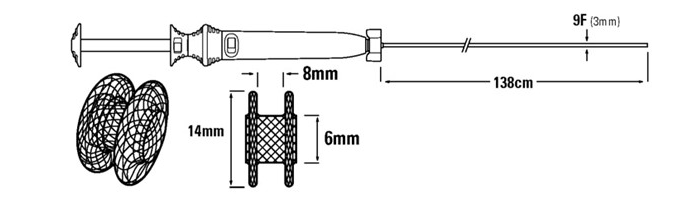
Source: FDA
Alarm reason
Boston Scientific has observed an increase in reports of difficulty with stent deployment and expansion during delivery.This issue may prolong the procedure time and require stent replacement; if the stent cannot be expanded, additional endoscopic or surgical intervention may be necessary.
As of December 23, 2025, the company has received 167 reports of serious injuries and 3 reports of deaths related to this issue.
Affected Product Information
Official announcements disclose that the following lots of AXIOS stents and electrocautery-enhanced delivery systems must be immediately discontinued and quarantined:
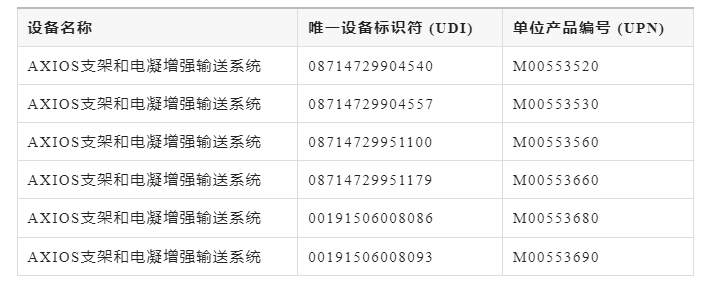
Immediate actions to take
Boston Scientific issued a letter to affected customers on December 19th, requesting the following:
Stop using, distributing, or selling the affected devices immediately. Remove them from inventory and isolate them until they are returned.
Post conspicuous notices in the relevant areas to ensure all operators are aware.
Please forward this notice to all relevant healthcare personnel within your institution and to other institutions that may have received this product.
Distributors must ensure that notifications are passed on to the end-users.
Use the AXIOS stent strictly according to the product instructions.
This issue only occurs during stent implantation and does not affect successfully implanted stents. Affected patients should continue standard treatment and follow-up.
FDA continues to evaluate this high-risk medical device issue and will keep the public informed as new information becomes available. Stakeholders are encouraged to monitor the official FDA website for the latest updates.
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

Key Players: The 10 Most Critical Publicly Listed Companies in Solid-State Battery Raw Materials
-

Vioneo Abandons €1.5 Billion Antwerp Project, First Commercial Green Polyolefin Plant Relocates to China
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics
-

Clariant's CATOFIN™ Catalyst and CLARITY™ Platform Drive Dual-Engine Performance
-

List Released! Mexico Announces 50% Tariff On 1,371 China Product Categories






