Fda approved: Portable Neonatal Incubator
In January 2026, UK newborn care medical technology company mOm Incubators announced that its core product, the Essential Incubator, had received 510(k) market clearance from the U.S. Food and Drug Administration (FDA), making it one of the few portable neonatal incubator systems to obtain U.S. regulatory approval.
This approval signifies that the device can officially enter the U.S. market for providing continuous and stable temperature management for premature infants and low birth weight newborns. The FDA's 510(k) clearance pathway indicates that the system has been determined to be substantially equivalent in safety and performance to legally marketed predicate devices, without requiring the more complex PMA approval process.

Neonatal Temperature Control Device for Non-NICU Settings
Essential Incubator falls under the Neonatal Incubator category, but it is positioned not as a large, stationary device in a traditional NICU, but rather as a portable temperature control system for the following scenarios:
Delivery Room Care
Primary care hospital without an independent NICU
Intrahospital transport and bedside nursing care
Rural and Remote Area Medical Institutions
Humanitarian Aid in Low-Resource Environments
Unlike traditional incubators primarily serving centralized NICUs, this system emphasizes continuous use from delivery to postpartum care, ensuring neonatal temperature stability while minimizing care interruptions caused by transport or equipment limitations.
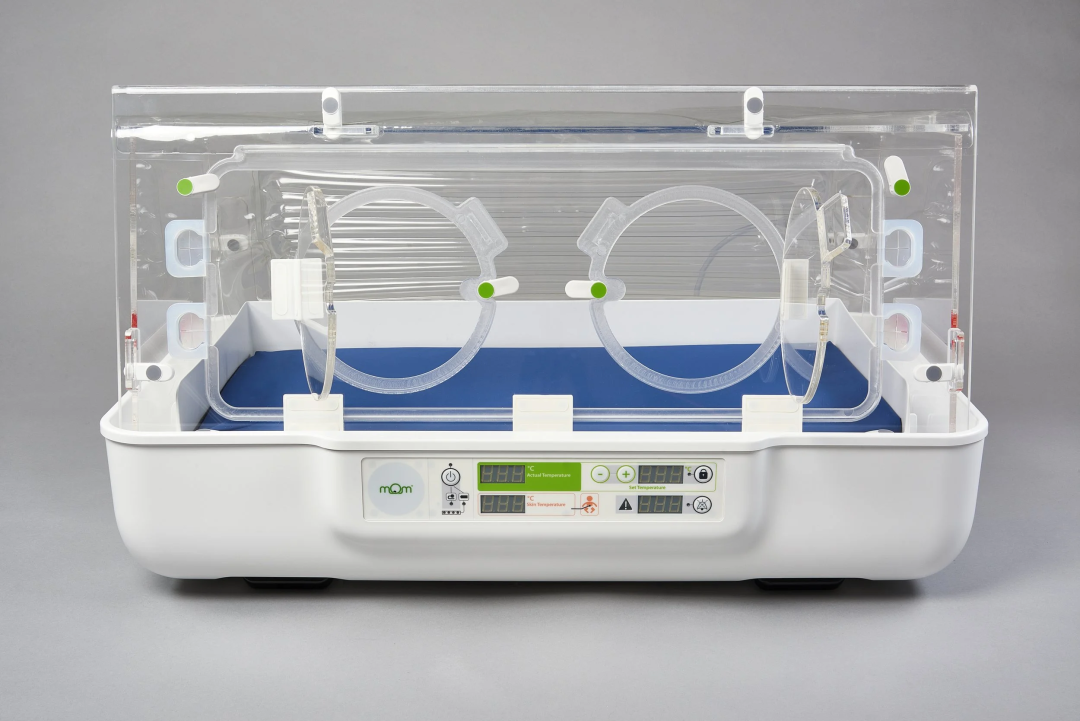
System design focusing on "Portability + Continuous Temperature Control"
From a technical standpoint, the core innovation of the Essential Incubator is not a breakthrough in a single performance metric, but rather a systemic restructuring of the newborn care process, primarily manifested in the following aspects:
Lightweight and portable design
With a total weight of approximately 20kg, significantly lower than the typical hundreds-of-kilograms structure of traditional incubators, it can be carried and quickly deployed by a single person, making it suitable for use in delivery rooms, patient rooms, and during transport.
2. Continuous constant temperature and multi-power supply support
The device features precise and stable temperature control and supports multiple power sources including AC power and battery. In the event of a power outage or during transport, the built-in battery can maintain stable temperature control for at least 1 hour, reducing the risk of hypothermia in newborns.
3. Open Architecture and Ease of Use
Adopting an open operating interface and modular component design facilitates quick access and cleaning/disinfection by medical staff, helping to lower the operational threshold and the risk of hospital-acquired infections.
4. Security and Compliance Fundamentals
Having passed medical electrical safety, biocompatibility, and performance testing in compliance with relevant international medical device standards, the system has obtained CE certification and FDA 510(k) clearance, providing a fundamental guarantee for its application under different regulatory systems.
5. Nursing Model Adaptation
The equipment supports delivery, postpartum care, and Family Integrated Care (FICare) models, allowing newborns to maintain closer and longer contact with their parents while receiving thermoregulatory protection.
Compliance expansion from Europe to the US
From a regulatory perspective, the mOm Essential Incubator's path to market is somewhat representative.
2022: Achieved CE certification and launched in the UK and European markets.
January 2026: Received FDA 510(k) clearance, entering the US market
FDA's choice of the 510(k) pathway over PMA implies that the core risks of the product are manageable and that its technological approach is considered an extension of the functionality of existing neonatal temperature control devices rather than a disruptive replacement.
From a market perspective, a significant number of maternity facilities in the United States lack comprehensive NICU facilities, and globally, there are still significant equipment gaps in premature infant care in primary hospitals, transport systems, and low-resource regions. Portable incubators, therefore, serve as a complementary solution to traditional stationary equipment in this context.

Enter the newborn care niche market with a single product.
Company Background
mOm Incubators was founded in 2014 in Nottingham, UK, by founder James Roberts. The company focuses on neonatal care, with a core principle of improving the care conditions for premature babies in non-ideal medical environments through more lightweight and easier-to-deploy device designs.
To date, the company has raised approximately $9.3 million in funding. It has a small team, but its product is already being used within the UK's NHS system and in some humanitarian settings. In early 2026, the company appointed Daniel Green as COO to strengthen commercialization and global market expansion capabilities.
The role of product in company strategy.
Essential Incubator is currently mOm's sole core commercialized product and a key vehicle for entering the neonatal care market. Following FDA approval, the product will serve as an important foundation for the company's expansion into the US market, while also providing regulatory endorsement for its continued deployment in Europe, Asia-Pacific, and humanitarian healthcare sectors.
# Conclusion: Regulatory Implementation and Practical Significance of Portable Temperature Control Devices
The FDA approval of the mOm Essential Incubator doesn't signify a fundamental shift in newborn care, but it does represent a clear trend: newborn temperature management is moving away from centralized, fixed locations towards a more flexible approach closer to the birthing site.
For newborn care systems, these devices primarily serve as "process fillers"; and for small and medium-sized medical technology enterprises, their regulatory pathways and market entry strategies provide a referential practical model for products with similar positioning.
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

Key Players: The 10 Most Critical Publicly Listed Companies in Solid-State Battery Raw Materials
-

Vioneo Abandons €1.5 Billion Antwerp Project, First Commercial Green Polyolefin Plant Relocates to China
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics
-

Clariant's CATOFIN™ Catalyst and CLARITY™ Platform Drive Dual-Engine Performance
-

List Released! Mexico Announces 50% Tariff On 1,371 China Product Categories






