Latest policy! 8 categories of high-end medical devices clearly accelerate approval
Recently,National Medical Products Administration(NMPA) released the "Priority Approval High-end Medical Device Catalogue (2025 Edition)", which includes8 types of high-end medical devicesIncluded in the priority approval scope. Unlike before, this list is not merely a "technical checklist," but rather resembles a clear regulatory statement:Under the premise of safety and effectiveness, strive for time and certainty for high-end technologies that are truly in a critical window period.。
The selected directions for these 8 types of products span multiple highly complex fields, including tumor radiotherapy, imaging equipment, interventional therapy, surgical robots, neuroregulation, and life support. They encompass frontier technologies that are still lacking domestically and mature areas with existing products but with clear clinical or technical boundaries.

"Priority approval" does not equate to lowering standards, but rather is a form ofChanges in the allocation methods of regulatory resources。
Under the current medical device registration system, high-end innovative devices often face challenges such as technical complexity, long clinical validation cycles, and high registration communication costs. By establishing a priority approval list for high-end medical devices, the signal released by the regulatory authorities is not one of "approval," but rather:Under the premise of meeting safety and effectiveness requirements, reduce unnecessary institutional time costs through early intervention, parallel review, and resource prioritization.。
Therefore, the catalog itself is not the end, but aThe starting point for technology to be included in the "priority review perspective."。
Overview of Eight Types of High-End Medical Devices
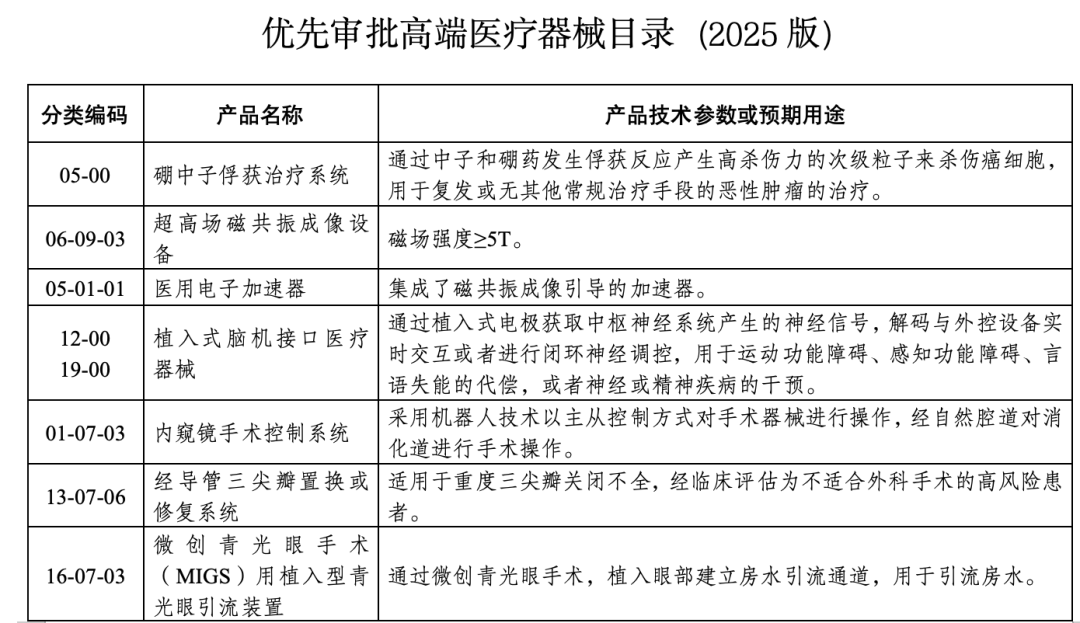

Boron Neutron Capture Therapy System (BNCT)
The system utilizes the capture reaction between neutrons and boron drugs to produce highly lethal secondary particles, which are used for malignant tumors that have recurred or lack conventional treatment methods. The difficulty lies not in a single device, but in...Neutron source, boron drugs, dosimetry, and the synergy of the overall treatment systemThe inclusion of this in the priority review list means that the regulatory level has begun to reserve a pathway for this highly systematic treatment technology.
Implantable Brain-Computer Interface Medical Device
Collecting or regulating central nervous system signals through electrode implantation for compensation of motor, sensory, speech dysfunction, or intervention in neuropsychiatric disorders. Priority approval does not mean "imminent market release," but rather...Establish a more predictable registration pathway for complex technologies and products requiring long-term follow-up.。
Minimally Invasive Glaucoma Surgery (MIGS) with Implantable Drainage Devices
Establishing an aqueous humor drainage channel inside the eye through minimally invasive methods to reduce intraocular pressure.
Transcatheter tricuspid valve replacement or repair system
There are already approved products domestically, but the current products are mostly used to alleviate tricuspid regurgitation and heart failure symptoms, and there are strict limitations on the anatomical conditions and disease stages of patients. The significance of expedited approval lies in providing...Next-generation products with broader indications and more refined structural design.Provide a faster review pace.
Endoscopic surgery control system
Robotic master-slave control is used to perform surgeries such as those in the digestive tract via natural orifices. Several products have already been approved, but there is still room for continuous iteration in terms of flexibility, precision operation capabilities, and the range of surgical procedures covered.
Medical Linear Accelerator (MRI-guided)
Integrating magnetic resonance imaging with radiotherapy accelerators for image-guided precision treatment. This approach demands higher capabilities in system integration and clinical workflow restructuring, and prioritized approval helps shorten the time for complex systems to enter clinical use.
Ultra-high field magnetic resonance imaging equipment (≥5T)
Belonging to the technological high ground in imaging equipment, it places extremely high demands on magnets, gradient systems, and safety control. Priority approval is more reflected in the requirements for.Institutional Support for High-End Imaging Capability Development。
Membrane oxygenator (for ECMO use)
The category explicitly defines localization conditions, such as domestically produced hollow fiber membrane modules or targeting newborns and children, reflecting the regulation on Continuous attention to the localization and controllability of critical life support equipment。
Why now?
From an industry perspective, the most core significance of this directory lies inWho can enter the verifiable, iterative clinical stage earlier?。
For enterprises, priority approval means:
Earlier technical communication with the review system.
Faster completion of key registration nodes.
Enter the commercialization or real-world data accumulation stage earlier.
For patients, it means potentialAdvanced treatment technologies become accessible earlier.。
More importantly, this "time value" can inversely support innovation. Earlier market entry and earlier commercial returns can continuously support the high-risk, high-investment research and development of medical devices.。
The common direction behind regulatory signals
If we observe these 8 types of products together, several common characteristics can be found:
The technical complexity is high, making it difficult to quickly convert solely through market spontaneity.
Most products have a clear unmet clinical need.
The research and validation cycle is long, requiring patience in terms of capital and regulations.
Once it enters clinical practice, it will have a substantial impact on the existing treatment pathways.
In other words, this is not a "trendy" list, but a list...Provide a list of institutional support for long-term technology pathways.。
Conclusion: Priority approval is not the end, but the starting line.
The release of the "Priority Approval List for High-end Medical Devices (2025 Edition)" does not imply that these technologies are about to be launched collectively, but rather signifies that they have been officially included.The track of collaborative advancement of regulation and innovation.。
The real point of continuous observation is in the coming years:
Which technologies have first completed clinical validation, which products have proven to be safe and effective in the real world, and which companies can transform the "time advantage" into long-term technological and industrial capabilities.
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

Key Players: The 10 Most Critical Publicly Listed Companies in Solid-State Battery Raw Materials
-

Vioneo Abandons €1.5 Billion Antwerp Project, First Commercial Green Polyolefin Plant Relocates to China
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics
-

Clariant's CATOFIN™ Catalyst and CLARITY™ Platform Drive Dual-Engine Performance
-

List Released! Mexico Announces 50% Tariff On 1,371 China Product Categories






