-
The "Twin Stars" of Regenerative Materials: CaHA and PLLA

Hydroxyapatite (CaHA) and poly-L-lactic acid (PLLA) are known as the "twin stars" of regenerative materials. These two biomaterials are widely used in the biomedical field and have become indispensable components in modern medicine. Whether for fract
Medical Materials Research Institute -
In-depth Analysis! Seven Major Orthopedic Biomedical Materials

Bone repair materials primarily refer to materials used to directly support, enhance, or replace damaged bone tissue. The design and application of these materials focus on the treatment and restoration of bone, such as fracture healing, bone defect
Bone Future -
Ultra-high molecular weight polyethylene: A miraculous material in the field of orthopedic implants

In today's continuously advancing orthopedic medical technology, the application of new materials brings more hope to patients. Among them, ultra-high molecular weight polyethylene (UHMWPEThe material performs excellently in the field of orthopedic i
CCME Intelligent Medical Devices -
Inventory of Bone Substitute Materials

Bone substitute materials, also known as bone graft substitutes or bone regeneration materials, are used in orthopedic and dental surgeries to replace natural bone. These materials are designed to support, enhance, or promote the regeneration and rep
Medical polymer materials -
Overview of the Application of Polycarbonate in the Medical Field

Polycarbonate (PC), due to its excellent physical and chemical properties (such as high transparency, high strength, heat resistance, biocompatibility, etc.), has widespread applications in the medical field.Medical devicesDisposable medical supplies
Medical Advanced Materials -
The world's first! "A medical device" has been approved by the FDA.

Recently, the U.S. Food and Drug Administration (FDA) approved a system for cleaning flexible endoscopes' complex internal channels for the first time. These channels are a stubborn source of hospital-acquired infections. This breakthrough was achiev
Pharmaceutical Intelligence Medical Device Data -
Medical-grade high-end chemical materials: current situation and future
Medical-grade chemical materials come in many types, primarily可分为高分子、金属、无机非金属及复合材料, and they are mainly applied in areas such as medical devices, drug carriers, implant equipment, and more. Note: The phrase "可分为高分子、金属、无机非金属及复合材料" is directly translat
Chemical Engineering Society -
【Medical Section】Modification Effects of Vacuum Plasma Cleaning Machines on Medical Material Surfaces
The polyurethane artificial blood vessels have shown localized 'snowflake-like' detachment of the anti-coagulation coating on their walls, which is no longer an isolated case. In the field of biomaterials, high-performance polymers such as polyuretha
Ples Electronic Technology -
PhD from the Chinese Academy of Sciences: Research and Development of Polyolefins and Their Modification Technologies for High-End Medical Device Applications
Speech TitleResearch and Development of Polyolefins and Their Modification Technologies for High-End Medical Devices ApplicationsGao MingDr. [Name], a senior engineer at the Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, ser
Polyolefin Person -
Co-extrusion films in medical applications.

Multilayer co-extruded films refer to films made from three or more types of plastic pellets or powders. These materials are melted and plasticized by multiple extruders and simultaneously extruded through a shared die head to produce the film. Multi
Medical polymer materials -
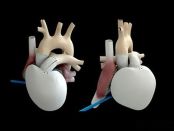
**Translated Content:** Heart Valve Material Analysis: Guarding the Heart's "Gate of Life" In the intricate world of medical device materials, the analysis of heart valve materials holds pivotal importance, especially as these components act as the vital gatekeepers to the heart — its "Gate of Life." This translation retains the essence and technical precision of the original Chinese text, making it accessible to English-speaking audiences engaged with cardiovascular medical device research and development.

Recently, cardiac valve repair system developer cardiac Dimensions announced that it has completed an oversubscribed Series E funding of $53 million (approximately 38.3 million RMB), with Ally Bridge Group leading the investment.In the field of medic
Medical Materials Research Institute -
Biomedical Materials — NC Membrane

In the wondrous world of biomedicine, NC membrane is like a sharp detective, using its unique "membrane power" to navigate every corner of precise diagnosis.Nitrocellulose membrane (abbreviated as NC membrane) has high protein binding capacity and hi
Medical polymer materials -
Introduction to Polymer Materials for Organ and Tissue Replacement

Skin, muscles, ligaments, cartilage, and blood vessels are all soft tissues, mainly composed of collagen. Collagen is the main component of connective tissue in mammals, making up about 30% of the body's proteins, with a total of 16 types, the most a
Medical polymer materials -
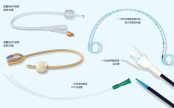
The Development of Percutaneous Coronary Intervention

Percutaneous Coronary Intervention (PCI) is a minimally invasive procedure used to treat coronary artery stenosis or occlusion. Its development history can be traced back to the 1970s, and after years of technological advancements and innovations, PC
Frontiers of High-Value Medical Consumables -
In-depth Analysis! Seven Types of Biomedical Materials for Orthopedics

Bone repair materials mainly refer to materials used for directly supporting, enhancing, or replacing damaged bones. The design and application of these materials focus on the treatment and recovery of bones, such as fracture healing, bone defect fil
Bone Future -
Industry Research | Application Prospects of PAEK Materials (PEKK, PEEK) in the Medical Field

Material Innovation: Performance Breakthrough of PAEKPAEK (Polyaryletherketone) as the fourth-generation medical polymer material, achieves three core breakthroughs through unique molecular design:Biomechanical adaptability: 3-4 GPa elastic modulus f
Advanced Medical Materials -
In which fields is medical PC used?
Sure, please provide the content that needs to be translated. It seems like the actual text is missing from your request. Once you provide the text, I can translate it for you while keeping the HTML tags and URLs intact.In the medical field, plastic
Souliao Network -
Evaluation and Prospects of the Wear Performance of Highly Crosslinked Polyethylene Acetabular Liners Containing Vitamin E

Total hip arthroplasty (THA) is one of the most successful orthopedic surgeries, bringing immeasurable benefits to patients with hip diseases around the world[1]. However, the biggest obstacle for patients with hip diseases on the road to recovery is
China NMPA
Most Popular
-

Key Players: The 10 Most Critical Publicly Listed Companies in Solid-State Battery Raw Materials
-

Vioneo Abandons €1.5 Billion Antwerp Project, First Commercial Green Polyolefin Plant Relocates to China
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics
-

Clariant's CATOFIN™ Catalyst and CLARITY™ Platform Drive Dual-Engine Performance
-

List Released! Mexico Announces 50% Tariff On 1,371 China Product Categories







